肝癌是世界第五大常见肿瘤,其病死率在肿瘤中居第3位[1]。射频消融术(radiofrequency ablation,RFA)做为治疗肝癌的常规方式之一,具有疗效显著,创伤小,可重复操作,安全及住院时间短等优点[2]。临床研究中,直径<3cm的肝癌的RFA治疗效果同手术切除相比其3年总体生存率无显著差异[3]。但RFA术也存在缺陷,主要表现在其一次性毁损的肿瘤体积有限,较大的肿瘤行RFA术后难以全部毁损,易产生残癌。有文献[4]报道指出,对于直径约5cm的肝癌行RFA后其残癌发生率可达30%~50%。因而,如何有效地增强RFA一次性的毁损体积,抑制残癌的生长已成为提高RFA疗效的关键。
基于RFA的基本原理,目前研究主要通过局部注射高渗盐水、乙醇、醋酸等来增加癌灶内离子浓度,从而增强RFA的毁损效应[5-7]。但是,局部注射难以使离子在癌灶内均匀分布,注射处离子浓度较高,而稍远处离子则不易弥散到,因而在行RFA时容易造成局部组织碳化而阻碍热量的传递,最终影响RFA疗效的提高。另外,局部注射时穿刺针的插入与拔出会增加癌细胞播散的几率。针对以上问题,本课题设想合成一种能直接通过血管注射,具有较好肿瘤靶向功能的纳米金(gold nanoparticles,GNPs)复合物,同时携带VEGFsiRNA基因。一方面GNPs能增加癌灶内离子浓度,且通过血管注射可使GNPs在肿瘤中分布相对均匀;另一方面VEGFsiRNA可抑制残癌生长,从而更好的增强RFA的损伤效应。
在本课题的前期研究中,课题组已成功合成GNPs-CPEI-G-PEG-TyrRGD/VEGFsiRNA复合物(TyrRGD-GNPs-VEGFsiRNA),合成方法参照文献[8]报道。其中GNPs为直径10~20 nm的金微小颗粒,作为增加肿瘤病灶内离子浓度的材料,增强RFA的直接损伤效应。环五肽RGD[cyclo-(Arg-Gly-Asp-D-Tyr-Lys),TyrRGD]作为靶向肿瘤细胞的分子材料,能与几乎只在肿瘤细胞以及肿瘤新生血管内皮细胞高度特异性表达的αvβ3特异结合[9]。VEGFsiRNA通过降解与之同源的mRNA,抑制肝癌中高表达的VEGF及其受体,从而抑制体内肝癌的生长[10-11]。
前期课题研究通过TyrRGD-GNPs-VEGFsiRNA复合物与肝癌细胞共培养,已证实该复合物与肝癌细胞的结合能力显著强于正常肝细胞,在30%浓度下对正常肝细胞无毒性作用;同GNPs相比,该复合物具有更好的离散性,且更容易与肝癌细胞相结合;在对离体猪肝行RFA试验中,也证明注射该复合物能显著增大RFA的毁损直径[12]。本文是在课题前期研究的基础上,进行TyrRGD-GNPs-VEGFsiRNA复合物的体内实验,探讨TyrRGDGNPs-VEGFsiRNA复合物是否可增强RFA对兔肝脏VX2肿瘤的毁损效应以及对残癌组织生长的影响。
1 材料与方法
1.1 主要材料与试剂
63只新西兰大白兔,雌雄不限,体质量2.0~2.5 kg,由第三军医大学大坪医院实验动物中心提供,动物饲养在西南医院动物实验室,所有动物实验均遵循第三军医大学动物管理委员会的规定。VX2实体瘤组织块购买于北京北纳创联公司,罗氏TUNEL凋亡试剂盒购买于德国罗氏公司(德国),TyrRGD-GNPs-VEGFsiRNA复合物由课题组提供,GNPs溶液购买于上海沪正纳米科技有限公司,戊巴比妥钠购买于北京索莱宝科技有限公司,DAB显色试剂盒购买于北京中杉生物技术有限公司,4%多聚甲醛购买于武汉博士德生物工程有限公司。射频治疗仪采用OLYMPUSCELON POWER射频消融系统,由西南医院肝胆外科提供,购买于北京贝恩医药科技开发有限责任公司。其他材料由西南医院肝胆外科实验室提供。
1.2 实验方法
1.2.1 建立VX2兔肝癌模型 VX2肿瘤是兔乳头状瘤经Shope病毒诱导而形成的一种可植入的鳞状细胞肿瘤,该肿瘤可在兔肝上生长并同人肝细胞癌有许多生物相似性,所以常作为研究肝癌介入治疗的理想动物模型。模型建立方法参照文献[13]。VX2肿瘤细胞系靠植入荷瘤兔后腿大腿肌肉中维持传代。动物由3%戊巴比妥钠(按1 mL/kg)经耳缘静脉麻醉。从荷瘤兔中收取鱼肉状的VX2新鲜组织,置于无菌生理盐水中备用。取剑突下腹部正中切口,逐层入腹,暴露肝左叶,并在其较厚处用眼科镊建一8 mm深窦道,将肿瘤组织切成1 mm3大小,植入窦道底部,海绵明胶封闭窦道口,关腹。3周后,通过超声观察VX2兔肝肿瘤生长状况,通过测量,肿瘤直径分布在1.8~2.3cm范围。
1.2.2 透射电镜观察标本 取6只VX2兔,分两组,每组3只。经耳缘静脉分别注射TyrRGD-GNPs-VEGFsiRNA复 合 物 0.2 mL,GNPs液 0.2 mL。48 h后处死动物,取肿瘤标本,经2.5%戊二醛保存后送学校电镜室制片,观察。
1.2.3 RFA操作 取30只VX2兔,分3组,每组10只。经耳缘静脉分别注射TyrRGD-GNPs-VEGFsiRNA复合物溶液,GNPs液及生理盐水各0.2 mL。48 h后,3%戊巴比妥钠麻醉、剃毛、消毒,固定于操作台上。超声定位肿瘤部位和进针路线,射频针穿刺至肿瘤中心部位,开始射频操作,起始功率设定为20 W,射频时间5 min。射频结束后,烧灼针道,退出射频针,观察有无出血。实验兔返回动物喂养室观察。
1.2.4 测量RFA毁损体积及残癌细胞凋亡 RFA治疗48 h后,每组各取10只实验兔,麻醉后取肿瘤标本,沿射频针道横截面切开肿瘤,测量毁损灶的长短径(长径a,短径b,单位:cm),并根据公式计算毁损体积:毁损体积=0.5×a×b2。残癌细胞凋亡测定按罗氏TUNEL试剂盒说明书进行。
1.2.5 生存时间测定 实验分3组,每组9只,即RFA联合TyrRGD-GNPs-VEGFsiRNA组,RFA联合生理盐水组及生理盐水组,饲养至其自然死亡,记录生存时间。
1.3 统计学处理
运用SPSS 18统计软件分析数据。定量数据采用均数±标准差(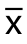 ±s)表示,多组间数据均数两两比较采用LSD-t检验,生存分析采用Kaplan-Meier检验,P<0.05为差异有统计学意义。
±s)表示,多组间数据均数两两比较采用LSD-t检验,生存分析采用Kaplan-Meier检验,P<0.05为差异有统计学意义。
2 结 果
2.1 TyrRGD-GNPs-VEGFsiRNA复合物具有较好的靶向性
透射电镜观察VX2肿瘤标本,TyrRGD-GNPs-VEGFsiRNA复合物在500 nm视野下出现的颗粒数为(14.2±2.6)颗,且分布较好;而GNPs在500 nm视野下颗粒数为0,两者有统计学差异(P<0.01)(图1)。
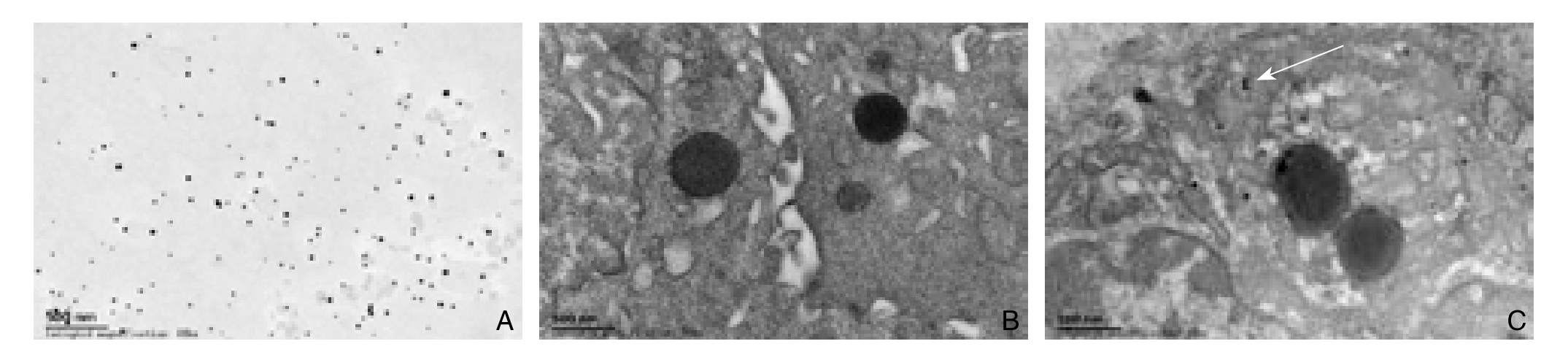
图1 电镜观察TyrRGD-GNPs-VEGFsiRNA复合物与GNPs在肿瘤细胞中的聚集(×50 000) A:电镜下TyrRGDGNPs-VEGFsiRNA复合物形态;B:GNPs组;C:TyrRGD-GNPs-VEGFsiRNA组
Figure 1 Gathering of TyrRGD-GNPs-VEGFsiRNA and GNPs in tumor tissue cells detected by electron microscope (×50 000)A: The morphology of TyrRGD-GNPs-VEGFsiRNA under electron microscope. B: GNPs group. C: TyrRGD-GNPs-VEGFsiRNA group
2.2 RFA治疗后各组毁损体积比较
VX2兔行RFA后,根据相应公式计算出其毁损体积。其中,注射TyrRGD-GNPs-VEGFsiRNA组毁损体积为(5.12±2.12)cm3;GNPs组为(1.78±0.46)cm3,生理盐水组为(1.49±0.44)cm3。TyrRGD-GNPs-VEGFsiRNA组的毁损体积明显大于GNPs组和生理盐水组,差异有统计学意义(P<0.01),GNPs组和生理盐水组间毁损体积比较无统计学差异(P>0.05)(图2)。另外,在RFA中由于其热效应而产生的气体在超声图像里表现为强烈的白色光团,其大小也可间接反映射频消融的毁损效应,TyrRGD-GNPs-VEGFsiRNA组的光团明显大于其余两组(图2)。
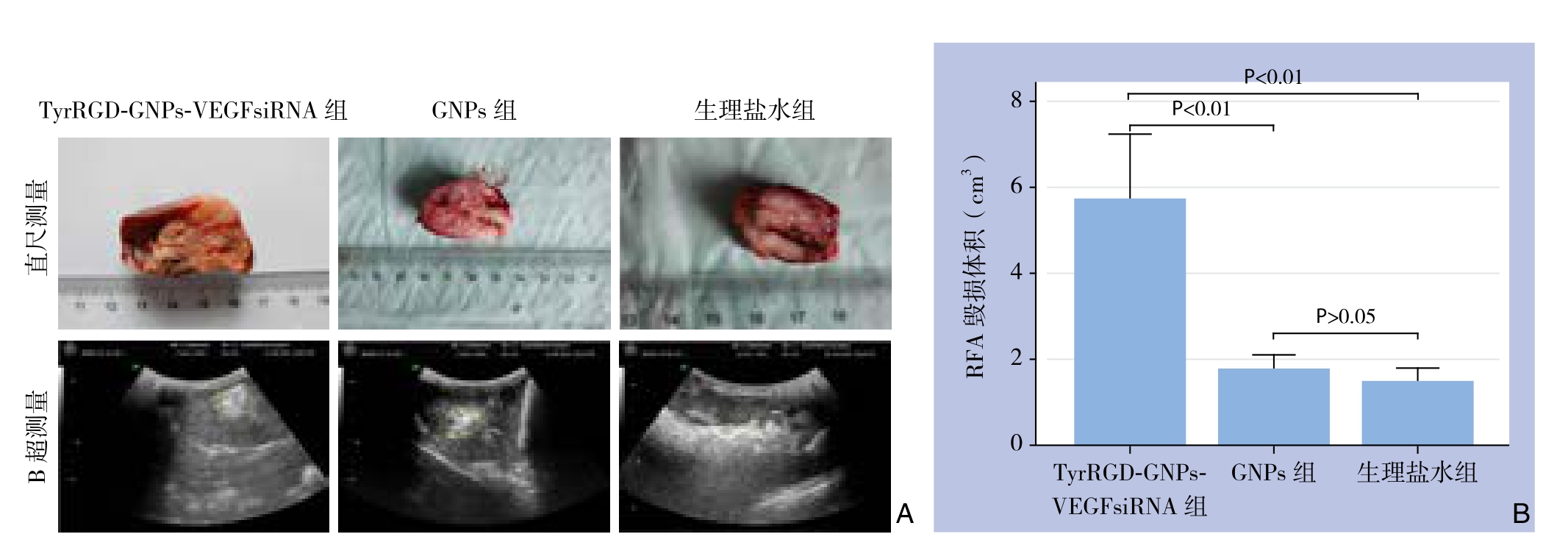
图2 RFA术后各组肿瘤毁损体积检测 A:通过直尺和B超分别测量各组肿瘤的毁损体积;B:各组毁损体积比较
Figure 2 Detection of ablation volume of the tumor of each group after RFA A: Measurement of damage volume by ruler and ultrasound;B: Comparison of ablation volumes among groups
2.3 HE染色及TUNEL法测凋亡
VX2兔行RFA后,肿瘤标本经HE染色可看到界限清楚的3个区域,即肿瘤完全坏死区、坏死周边区及残癌区。经TUNEL法染色后,坏死细胞表现为棕色(图3)。TyrRGD-GNPs-VEGFsiRNA组中残癌区域可观察到片状棕黄色细胞坏死区,而在GNPs组和生理盐水组相应区域则未见明显的细胞坏死区域(图3)。荧光显微镜100倍视野下计数残癌区域内凋亡细胞,其中TyrRGDGNPs-VEGFsiRNA组凋亡细胞数为(111.7±12.2)个,GNPs组为(36.3±3.8)个,生理盐水组为(34.7±6.1)个。TyrRGD-GNPs-VEGFsiRNA组残癌区凋亡细胞显著多于其余两组,差异有统计学意义(P<0.01),GNPs组和生理盐水组残癌区域内凋亡细胞数比较无统计学差异(P>0.05)。
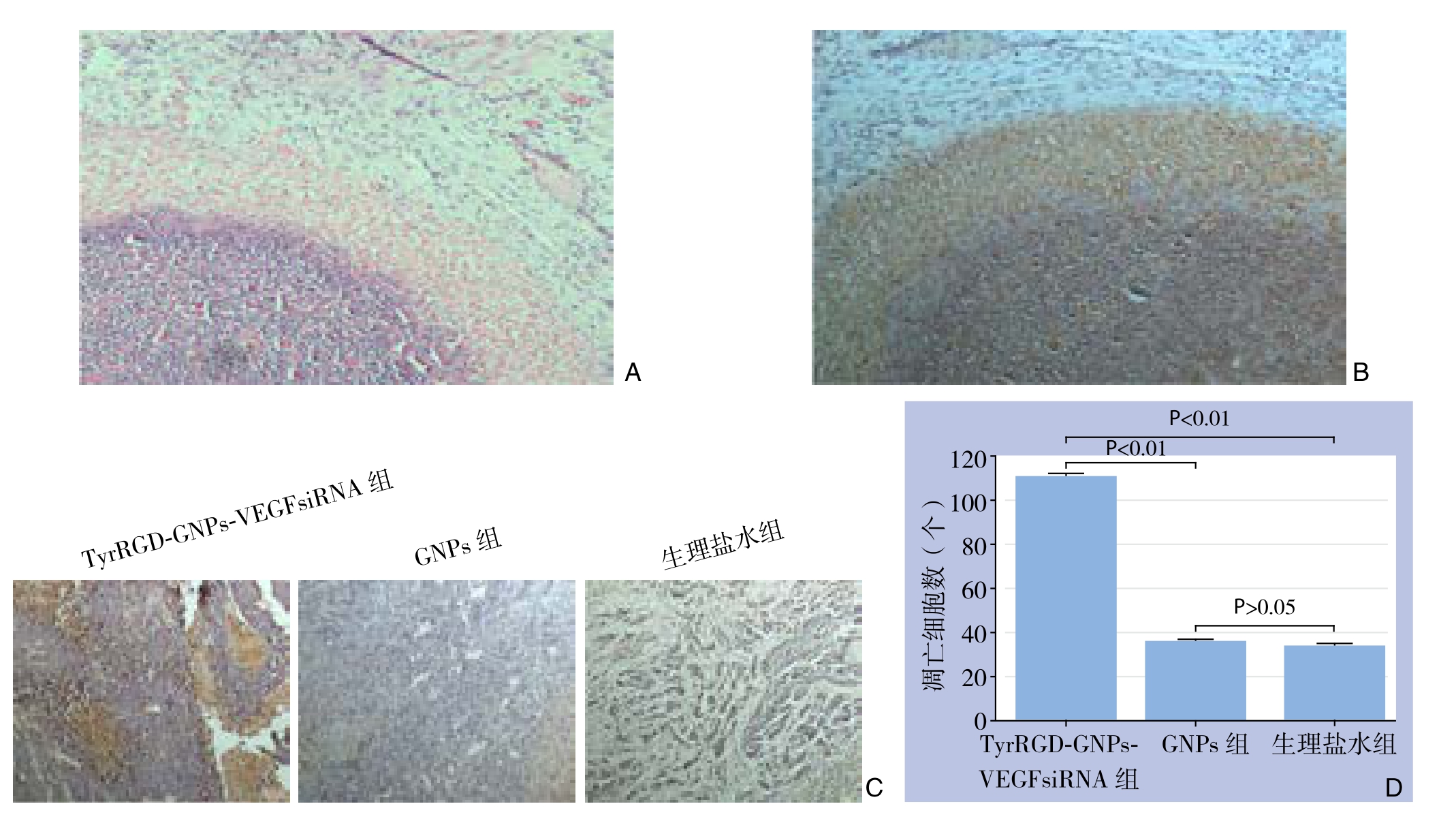
图3 各组残癌细胞凋亡检测 A:HE染色(×40);B:TUNEL染色(×40);C:TUNEL染色(×100);D:凋亡细胞计数
Figure 3 The apoptosis detection in the residual cancer cells A: HE staining (×40); B: TUNEL staining (×40); C: TUNEL staining(×100); D: Counts of apoptosis cells
2.4 生存时间
本研究中自然死亡的VX2兔共计27只,以3个月为观察期限,动物死亡率100%。饲养过程中只在RFA治疗前分别注射了TyrRGD-GNPs-VEGFsiRNA溶液及生理盐水。其中RFA联合TyrRGD-GNPs-VEGFsiRNA组的9只,平均生存时间70.9 d,其中6只有广泛的腹腔转移,转移率54%;RFA联合生理盐水组的9只,平均生存时间51.2 d,全部有腹腔转移;生理盐水组的9只,平均生存时间43.9 d,全部有腹腔转移。RFA联合TyrRGD-GNPs-VEGFsiRNA组生存时间明显长于RFA联合生理盐水组和生理盐水组(P<0.01),RFA联合生理盐水组的生存时间也长于生理盐水组(P<0.01)。生存曲线见图4。
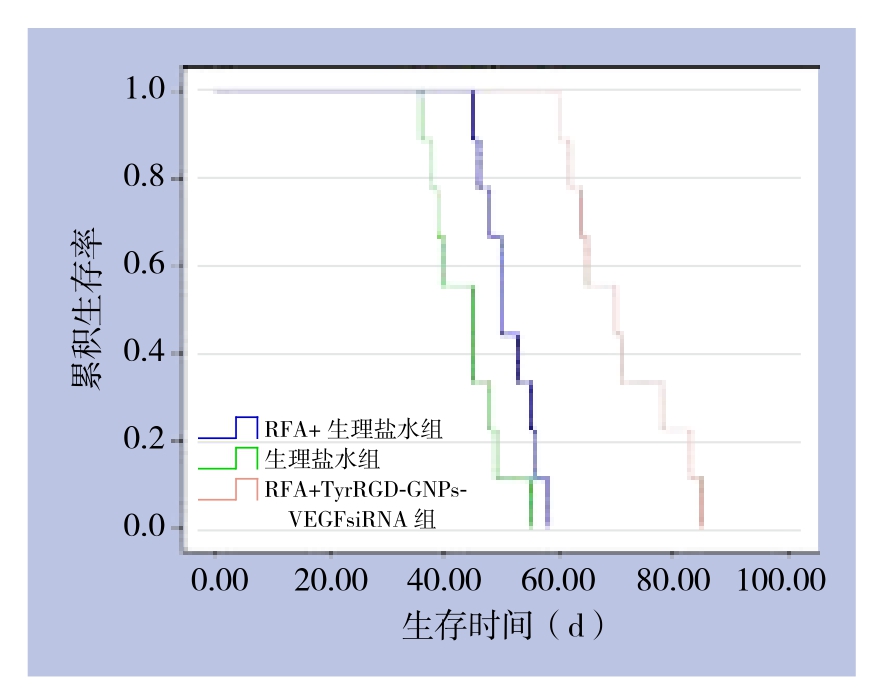
图4 各组兔生存时间比较
Figure 4 Comparison of survival time among groups of rabbits
3 讨 论
RFA是临床常用于治疗肝癌(包括原发性和继发性)以及其他实体肿瘤的微创治疗方法[14]。目前,RFA技术的主要缺陷在于其能够有效毁损肿瘤的体积有限[15-17]。研究[14,16,18]报道对于直径>5cm的肿瘤,RFA治疗后残癌发生率在10%~40%。Gannon等[19]研究证明,GNPs能够增强RFA对肝癌细胞的毁损效应,扩大毁损体积,且毁损效应随着GNPs浓度增加而增强,此外已证实进入细胞内的GNPs对细胞无毒性作用。同时多项研究表明GNPs可以偶联肿瘤特异性标志物或肿瘤相关的靶向分子(如:抗体、多肽、药物)而靶向体内的恶性肿瘤细胞,从而使体内的实体肿瘤得到有效可行的治疗。本课题组合成的TyrRGD-GNPs-VEGFsiRNA复合物,即是肿瘤靶向分子TyrRGD偶联GNPs颗粒,然后GNPs颗粒再偶联能促使肿瘤细胞凋亡的VEGFsiRNA而得[12]。本研究发现TyrRGD-GNPs-VEGFsiRNA复合物在VX2兔肝肿瘤组织内的聚集性明显优于单纯的GNPs颗粒。在注射该复合物后,RFA对肿瘤的毁损效应显著增强,不仅毁损体积明显增大,毁损程度更加彻底,且在一定程度上也促进了残癌细胞的坏死,降低肿瘤转移率,延长动物生存期。该结果与高勇等[20]报道的Tyr-RGD靶向的siRNA注射入小鼠体内24 h后,主要在肿瘤组织内分布一致;与Pedro等[21]报道的GNPs-TNFα能增强RFA对肾肿瘤毁损效应以及Raskopf等[11]报道的siRNA-VEGF能降低肿瘤微血管密度,抑制体内肿瘤生长的结果基本一致。
TyrRGD-GNPs-VEGFsiRNA复合物同单纯的GNPs相比较能好的被肿瘤细胞摄取,可能同以下因素有关。首先,TyrRGD能与肿瘤新生血管内皮细胞高表达的αvβ3特异结合,具有靶向作用[22];其次,TyrRGD可以促进肿瘤细胞对GNPs颗粒的摄取,并且可以包裹在GNPs表面,防止血清蛋白与其黏附,从而防止聚集[23];最后,GNPs经PEG功能修饰后,其性质更加稳定,不易聚集成团,同时也增强了细胞对其摄取能力[24-25]。实验中,TyrRGD-GNPs-VEGFsiRNA复合物组肿瘤毁损体积显著强于GNPs组和生理盐水组,而GNPs组同生理盐水组相比则无统计学差异,同时,电镜结果也表明只在TyrRGD-GNPs-VEGFsiRNA复合物组观察到GNPs颗粒,而GNPs组则无。这表明单纯的GNPs颗粒经静脉血管注射后无法有效的在动物肿瘤中聚集,而TyrRGD-GNPs-VEGFsiRNA复合物中的GNPs颗粒经TyrRGD靶向作用及PEG的功能修饰后可有效的到达动物肿瘤部位,从而实现增强RFA毁损效应的目的。
众所周知,索拉非尼可通过抑制包括VEGF在内的多个靶点发挥治疗肝癌的作用,GNPs也是良好的药物载体,理论上GNPs是可以结合索拉非尼治疗肝癌的。不过,在Simon等[26]的研究中,GNPs依靠疏水作用与索拉非尼结合,其结合率仅有10%。因此,若用索拉非尼替换复合物中的VEGFsiRNA发挥治疗肝癌的作用,仍需寻求其与GNPs有效的结合方式。
本研究初步证实了TyrRGD-GNPs-VEGFsiRNA复合物在增强RFA对VX2兔肝肿瘤损伤效应中的积极作用,但是肝脏中VEGF的表达是否被TyrRGD-GNPs-VEGFsiRNA抑制以及TyrRGD-GNPs-VEGFsiRNA复合物在动物体内分布及代谢情况则缺乏相关指标检测。对此,在本课题后期研究过程中可以通过Western blot检测各组中VEGF的表达情况;同时增添裸鼠成瘤模型,通过同位素示踪法或荧光标记法检测TyrRGDGNPs-VEGFsiRNA复合物在动物体内的整体分布及代谢情况,从而使本研究项目更加完整充实。
参考文献
[1] Amarapurkar D, Han KH, Chan HL, et al. Application of surveillance programs for hepatocellular carcinoma in the Asia-Pacific Region[J]. J Gastroenterol Hepatol, 2009, 24(6):955–961.doi: 10.1111/j.1440–1746.2009.05805.x.
[2] Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review[J]. Ann Surg, 2009, 249(1):20–25. doi: 10.1097/SLA.0b013e31818eec29.
[3] Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma[J]. J Hepatol, 2012, 57(4):794–802.doi: 10.1016/j.jhep.2012.05.007.
[4] Kudo M. Radiofrequency ablation for hepatocellular carcinoma:updated review in 2010[J]. Oncology, 2010, 78 (Suppl 1):113–124.doi: 10.1159/000315239.
[5] Sun Y, Cheng W, Han X, et al. In vivo experimental study on the effects of fluid in increasing the efficiency of radiofrequency ablation[J]. Asian Pac J Cancer Prev, 2014, 15(14):5799–5804.
[6] 陈敏, 高峻, 马宽生, 等. 射频消融联合醋酸加氯化钠对消融体积影响的临床研究[J]. 第三军医大学学报, 2007, 29(21):2089–2091.doi:10.3321/j.issn:1000–5404.2007.21.020.Chen M, Gao J, Ma KS, et al. Influence of radiofrequency ablation combined with acetic acid plus hypertonic saline injection on destructed volume of liver tumor[J]. Journal of third military medical university, 2007, 29(21):2089–2091. doi:10.3321/j.issn:1000–5404.2007.21.020.
[7] 范林军, 马宽生, 何振平, 等. 射频消融联合肝动脉及选择性门静脉栓塞治疗大肝癌[J]. 第三军医大学学报, 2002, 24(4):447–449.doi: 10.3321/j.issn:1000–5404.2002.04.024.Fan LJ, Ma KS, He ZP, et al. Treatment of large hepatocarcinoma with radiofrequency ablation combined with vascular embolization[J]. Acta Academiae Medicinae Militaris Tertiae, 2002,24(4):447–449. doi: 10.3321/j.issn:1000–5404.2002.04.024.
[8] Lee Y, Lee SH, Kim JS, et al. Controlled synthesis of PEI-coated gold nanoparticles using reductive catechol chemistry for siRNA delivery[J]. J Control Release, 2011, 155(1):3–10. doi: 10.1016/j.jconrel.2010.09.009.
[9] Kanchanawong P, Shtengel G, Pasapera AM, et al. Nanoscale architecture of integrin-based cell adhesions[J]. Nature, 2010,468(7323):580–584. doi: 10.1038/nature09621.
[10] Song WJ, Du JZ, Sun TM, et al. Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery[J]. Small, 2010,6(2):239–246. doi: 10.1002/smll.200901513.
[11] Raskopf E, Vogt A, Sauerbruch T, et al. siRNA targeting VEGF inhibits hepatocellular carcinoma growth and tumor angiogenesis in vivo[J]. J Hepatol, 2008, 49(6):977–984. doi: 10.1016/j.jhep.2008.07.022.
[12] 熊青, 邹孟达, 邓青松, 等. 载VEGF siRNA环五肽RGD偶联纳米金颗粒的基本特性及在射频消融中的应用[J]. 中国普通外科杂志, 2015, 24(1):45–51. doi: 10.3978/j.issn.1005–6947.2015.01.009.Xiong Q, Zou MD, Deng QS, et al. General properties and application of VEGF siRNA-loaded RGD-conjugated gold nanoparticles in radiofrequency ablation[J]. Chinese Journal of General Surgery, 2015, 24(1):45–51. doi: 10.3978/j.issn.1005–6947.2015.01.009.
[13] Ke S, Ding X M, Kong J, et al. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma[J]. J Transl Med, 2010, 8:73. doi:10.1186/1479–5876-8–73.
[14] Haemmerich D, Laeseke PF. Thermal tumour ablation: devices,clinical applications and future directions[J]. Int J Hyperthermia,2005, 21(8):755–760.
[15] Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients[J]. Ann Surg, 2004, 239(4):450–458.
[16] Gannon CJ, Curley SA. The role of focal liver ablation in the treatment of unresectable primary and secondary malignant liver tumors[J]. Semin Radiat Oncol, 2005, 15(4):265–272.
[17] N'Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis[J].Hepatology, 2009, 50(5):1475–1483. doi: 10.1002/hep.23181.
[18] Bleicher RJ, Allegra DP, Nora DT, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned[J]. Ann Surg Oncol, 2003, 10(1):52–58.
[19] Gannon CJ, Patra CR, Bhattacharya R, et al. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells[J]. J Nanobiotechnology, 2008, 6:2. doi: 10.1186/1477–3155-6–2.
[20] 高勇, 何胜利, 钟高仁, 等. 血管内皮细胞靶向性Tyr-RGD-PEGPEI纳米基因载体的合成及其生物特性的初步研究[J]. 药学学报, 2009, 44(9):1034–1039.Gao Y, He SL, Zhong GR, et al. Vascular endothelial cells targeted Tyr-RGD-PEG-PEI nano-drug synthesis and its biological activity[J]. Acta Pharmaceutica Sinica, 2009, 44(9):1034–1039.
[21] Pedro RN, Thekke-Adiyat T, Goel R, et al. Use of tumor necrosis factor-alpha-coated gold nanoparticles to enhance radiofrequency ablation in a translational model of renal tumors[J]. Urology, 2010,76(2): 494–498. doi: 10.1016/j.urology.2010.01.085.
[22] Graf N, Bielenberg DR, Kolishetti N, et al. alpha(V)beta(3)integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug[J]. ACS Nano, 2012, 6(5):4530–4539.doi: 10.1021/nn301148e.
[23] Yang C, Uertz J, Yohan D, et al. Peptide modified gold nanoparticles for improved cellular uptake, nuclear transport, and intracellular retention[J]. Nanoscale, 2014, 6(20):12026–12033. doi: 10.1039/c4nr02535k.
[24] Guduru R, Liang P, Runowicz C, et al. Magneto-electric nanoparticles to enable field-controlled high-specificity drug delivery to eradicate ovarian cancer cells[J]. Sci Rep, 2013, 3:2953.doi: 10.1038/srep02953.
[25] Dixit V, Van den Bossche J, Sherman DM, et al. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells[J]. Bioconjug Chem, 2006, 17(3):603–609.
[26] Simon T, Tomuleasa C, Bojan A, et al. Design of FLT3 Inhibitor -Gold Nanoparticle Conjugates as Potential Therapeutic Agents for the Treatment of Acute Myeloid Leukemia[J]. Nanoscale Res Lett,2015, 10(1):466. doi: 10.1186/s11671–015-1154–2.
