胃肠道间质瘤(gastrointestinal stromal tumor,GIST)是人类消化道中最常见的间充质肿瘤[1],被认为源于Cajal或其前体的间质细胞[2]。GIST可以出现在胃肠道管壁的任何地方,但在胃中尤其常见(60%~70%)[3-4]。存在于胃的称为胃的GIST,一般简称为胃间质瘤。在过去,开放手术或腹腔镜楔形切除术用来治疗胃间质瘤[5-6]。然而,内窥镜技术的快速发展提供了另一种新的治疗方法。90年代,日本将内镜黏膜下剥离术(endoscopic submucosal dissection,ESD)应用于临床,逐步替代内镜下黏膜切除术(endoscopic mucosal dissection,EMR)用于治疗一些消化道病变。较EMR而言,ESD可对>2 cm的消化道黏膜及黏膜下层的病灶或者溃疡型病灶进行黏膜下剥离,获得完整的组织学标本,从而准确评估病灶切缘是否有肿瘤浸润,有利于后续治疗方案的制定[7-9]。ESD的并发症主要是出血和穿孔[10]。术中出血比较常见,可以通过电凝、钛夹夹闭血管等方法成功止血[11]。研究[11-17]认为,ESD术后出血率为1.8%~8.2%;Takizawa等[18]研究报道预防性凝固创面可见血管可以有效降低术后出血率,但术后出血率仍为3.1%。所以ESD术后出血的危险因素仍需要进一步研究。ESD术后出血的危险因素,目前国内外报道结果不尽一致,尚无相关危险因素的一致结论[11, 14, 19-21],且国内外对胃间质瘤ESD术后出血的危险因素研究较少。因此,本研究旨在探讨胃间质瘤ESD术后出血的危险因素,为良好预防胃间质瘤ESD术后出血提供参考。
1 资料与方法
1.1 一般资料
收集武汉大学人民医院2011年2月—2017年5月胃间质瘤经ESD治疗患者相应的临床病历资料、内镜资料以及病理资料。排除标准:⑴ 资料不全的患者;⑵ 未顺利完成ESD治疗的患者;⑶ 瘤体个数≥2的患者;⑷ 有肿瘤转移的患者。
1.2 器材物品准备
电子胃镜(G I F-H 2 6 0 Z,G I F-H 2 6 0 J Olympus,Japan),Olympus公司的ESG100、PSD-60高频电源设备,EVIS 260主机,含NBI功能及副注水系统,圆筒形透明帽和ST帽,钩形刀(hook knife),KD-611L IT刀、针形切开刀(needle knife)、NM-4L-1注射针、FD-410LR热活检钳、ERBE ICC200高频电刀,Olympus金属钛夹、电凝止血钳、靛胭脂、复方碘液、10%甘油和5%果糖混合的甘油果糖溶液等。
1.3 操作方法
术前常规禁食12 h,禁水6 h。气管插管全麻麻醉成功后,患者取左侧卧位,自患者口腔顺利置入胃镜。探查并找到病灶。于病灶边缘0.5 cm处电凝标记切除范围。病灶黏膜下注射甘油果糖溶液。沿标记点外缘应用针形切开刀及IT刀切开周围全部黏膜。剥离器械沿黏膜下层剥离病变。剥离的肿瘤取出后测量大小、拍照,福尔马林固定后送病理学检查。
124例患者经ESD治疗后均行胃肠持续减压,根据引流胃液量、胃液颜色及患者恢复情况酌情拔除胃管,卧床休息,抑酸护胃,营养支持等对症治疗。术后第1天复查血常规等。对于术后胃管引流出血性液体较多、呕血、黑便及便血者立即急诊胃镜检查。术后出现腹痛加重,腹部出现压痛及反跳痛者复查腹部立位X线,发现腹腔游离气体,立即行手术修补穿孔。
1.4 资料收集
收集患者的临床、内镜、病理资料,统计患者的性别、年龄、超声胃镜诊断、瘤体最大直径、肿瘤部位、肿瘤来源、瘤体个数、淋巴结转移、瘤体生长方式、手术持续时间、术后腹痛持续时间,是否合并糖尿病、高血压、肝硬化、冠心病、陈旧性脑梗塞、肾功能不全、房颤基础疾病病史等。
1.5 统计学处理
采用SPSS 20.0统计软件进行分析,计量资料以均数±标准差(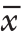 ±s)表示,胃间质瘤ESD术后出血相关危险因素的分析采用单因素及多因素非条件Logistic回归分析,OR>1、P<0.05为危险因素。
±s)表示,胃间质瘤ESD术后出血相关危险因素的分析采用单因素及多因素非条件Logistic回归分析,OR>1、P<0.05为危险因素。
2 结 果
2.1 一般情况
124例患者,男5 1例,女7 3例;年龄(53.532±10.358)岁,年龄范围29~79岁;病灶均为单发,无肿瘤转移;瘤体最大直径(1.262±0.576)cm,直径范围0.5~2.5 cm。合并高血压病11例,糖尿病13例,肝硬化4例,冠心病8例,陈旧性脑梗塞3例,肾功能不全2例,房颤5例,无妊娠患者。手术持续时间(38.484±9.507)min,范围24~61 min;术后腹痛持续时间(3.3 1 5±1.03 9)d,范围2~6 d。病灶位于贲门3例,胃底8 1例,胃体3 2例,胃窦7例;外生性生长2例,内生性生长120例,混合性生长2例;瘤体来源于黏膜层2例,黏膜下层120例,肌层2例。
2.2 胃间质瘤ESD术后出血的危险因素
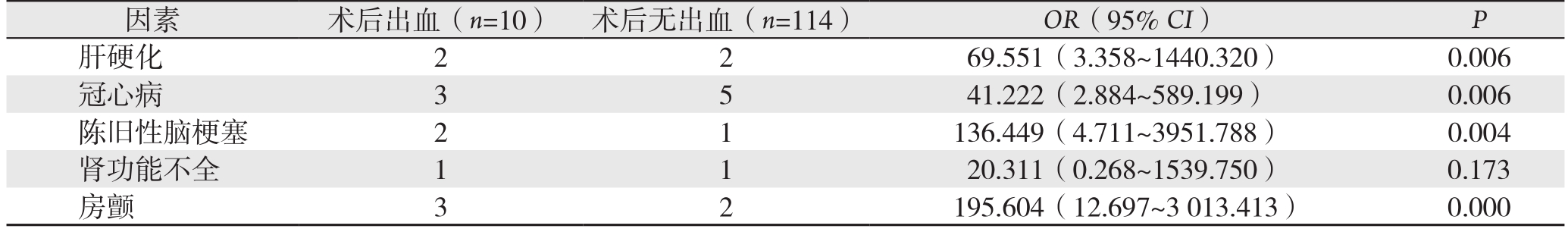
纳入研究的124例患者,有10例(8.06%)发生ESD术后出血。单因素Logistic回归分析结果表明,胃间质瘤ESD术后出血与肝硬化、冠心病、陈旧性脑梗塞、房颤病史有关(OR>1,P<0.05),而与患者的性别、年龄、病变部位、瘤体大小、瘤体生长方式、手术操作时间、术后腹痛持续时间、肿瘤来源无关(均P>0.05)(表1)。进一步行多因素Logistic回归分析表明,肝硬化、冠心病、陈旧性脑梗塞、房颤病史是胃间质瘤ESD术后出血的独立危险因素(OR>1,P<0.05)(表2)。
表1 胃间质瘤ESD术后出血的危险因素单因素Logistic回归分析
Table 1 Univariate Logistic regression analysis of risk factors for hemorrhage after ESD for gastric GIST
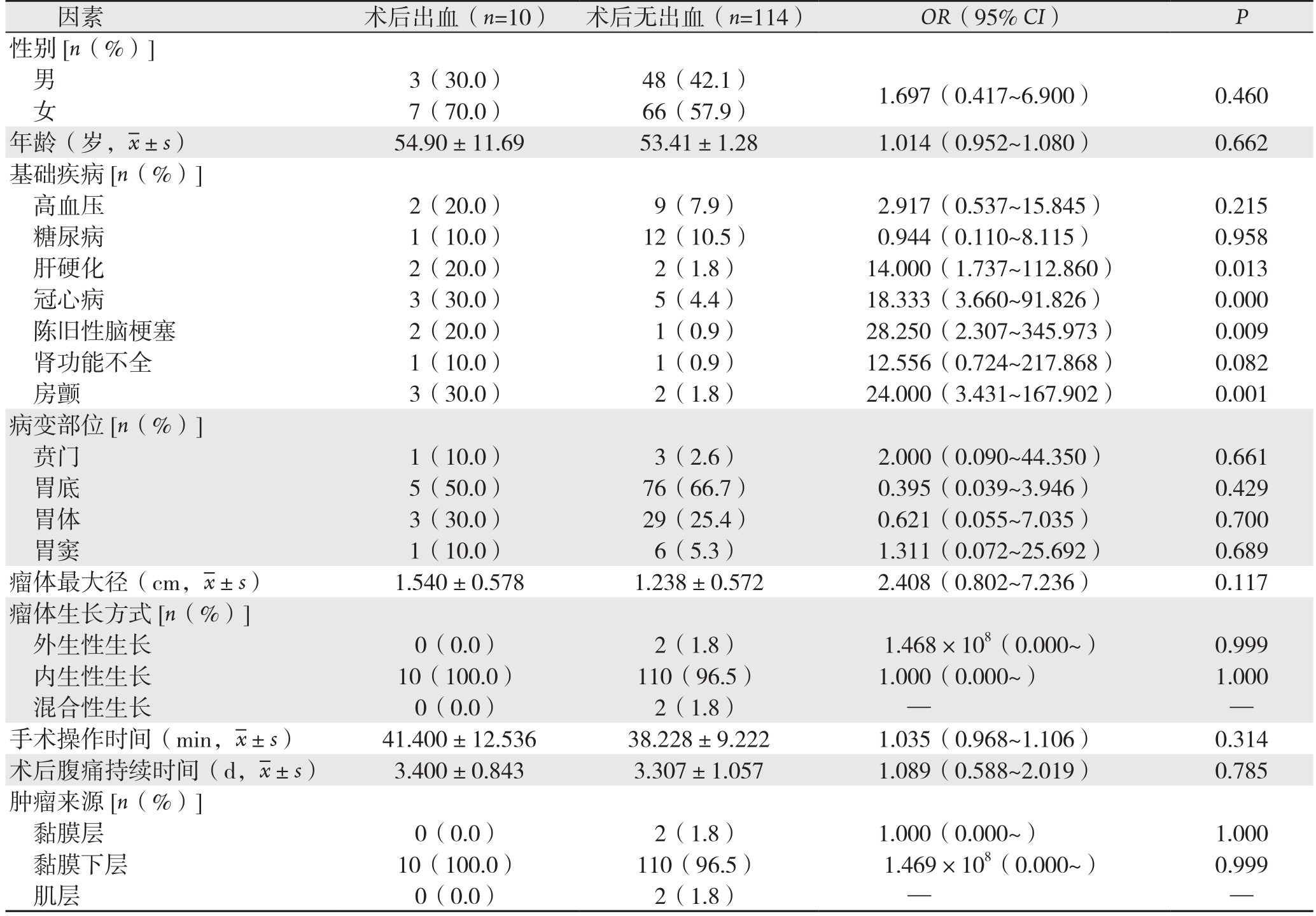
表2 胃间质瘤ESD术后出血的危险因素多因素Logistic回归分析
Table 2 Multivariate Logistic regression analysis of risk factors for hemorrhage after ESD for gastric GIST
3 讨 论
随着内镜技术的发展,很多过去由外科手术治疗的胃肠道肿瘤可以通过内镜切除。对于大多数消化道肿瘤如无转移的早期胃癌、GIST等均可行ESD治疗,且其治疗效果与外科手术相当[22]。已有研究[11-17]报道ESD术后出血率为1.8%~8.2%,本研究胃间质瘤ESD术后出血率为8.06%,与文献报道大体相似。Kim等[23]认为ESD术后出血的唯一危险因素是病灶大小。Mannen等[12]和Okada等[14]也提出大的病灶是ESD术后出血的危险因素。本研究无论从单因素回归分析还是从多因素回归分析,结果提示病灶大小与胃间质瘤ESD术后迟发性出血相关性无统计学意义(P>0.05),原因可能是本研究中胃间质瘤经ESD治疗的患者肿瘤直径均≤2.5 cm。这与Park等[24]的观点相似,他们认为,病灶>40 mm是ESD术后出血的危险因素。
Akasaka等[21]对一个大样本进行多因素回归分析,认为操作时间是胃ESD术后出血的独立危险因素。Toyokawa等[20]研究认为术后出血的主要危险因素是操作时间和年龄>80岁。他们认为:操作时间长可能会导致形成溃疡和其他不良反应;年纪大的患者易伴发基础病及机体基础情况较差。本研究经Logistic回归检验,结果提示,手术操作时间不是ESD术后出血的独立危险因素(P>0.05)。原因可能是胃间质瘤包膜较完整,且本研究中瘤体直径均≤2.5 cm,因此所需手术操作时间较短。
Miyahara等[25]研究发现位于远端胃的病灶是ESD术后出血的危险因素。而 Park等[24]则认为位于近端胃的病灶是ESD术后出血的危险因素。Yoon等[26]研究认为位于近端胃的病灶和胃后壁的病灶ESD操作所需时间延长。Chung等[27]认为位于近端胃的病灶,在ESD操作时,电刀难以完全到达粘膜下层及病灶基底,因此电刀不能按原计划控制切除方向和深度。另外,胃上部血管较下部血管直径粗,而且数量多[28-29]。本研究结果提示病变部位不是ESD术后出血的独立危险因素(P>0.05),可能的原因是本研究中胃间质瘤的好发部位刚好与易出血部位重合(都是近端胃)。
本研究结果显示,肝硬化、冠心病、陈旧性脑梗塞、房颤病史与胃间质瘤ESD术后出血明显有关(OR>1,P<0.05)。这可能的原因是这类基础疾病本身及这类疾病的治疗影响了凝血、止血功能,长期的抗凝、抗血小板治疗增加了胃间质瘤ESD术后出血的危险。Cho等[30]研究发现持续使用抗凝药会增加ESD术后出血的风险。Ojiam等[31]也报道了ESD术后出血率为3.9%,主要与抗凝药、血液透析、抗血小板药及降压药的使用相关。遗憾的是,本研究没有分析抗凝药、抗血小板药的应用对胃间质瘤ESD术后出血的影响。因此,笔者后续将进一步研究抗凝药、抗血小板药的应用对胃间质瘤ESD术后出血的影响。
此外,术者的操作经验也是促使胃间质瘤ESD术后出血发生的危险因素之一[32],理应考虑到研究分析当中;由于我院消化内镜中心所有ESD操作均由几位经验丰富的内镜操作医师完成,ESD顺利完成量均超过百例,组间无明显差异,因此也未作为研究因素进行分析。
综上所述,肝硬化、冠心病、陈旧性脑梗塞、房颤病史为胃间质瘤ESD术后出血的危险因素,所以对于该类患者术中与术后应给予重视。ESD操作难度大,手术时间长等出血风险较大的患者,应该加强术后管理,降低术后出血率。同时应该优化术前评估、术前准备以降低术后出血率。因为本研究属于回顾性研究,存在一定的局限性,所以尚需进一步研究其他相关因素。
参考文献
[1]Oppelt PJ,Hirbe AC,Van Tine BA.Gastrointestinal stromal tumors (GISTs):point mutations matter in management,a review[J].J Gastrointest Oncol,2017,8(3):466–473.doi:10.21037/jgo.2016.09.15.
[2]Belinsky MG,Cai KQ,Zhou Y,et al.Succinate dehydrogenase deficiency in a PDGFRA mutated GIST[J].BMC Cancer,2017,17(1):512.doi:10.1186/s12885–017–3499–7.
[3]Bertolini V,Chiaravalli AM,Klersy C,et al.Gastrointestinal stromal tumors--frequency,malignancy,and new prognostic factors:the experience of a single institution[J].Pathol Res Pract,2008,204(4):219–233.doi:10.1016/j.prp.2007.12.005.
[4]Yu C,Liao G,Fan C,et al.Long-term outcomes of endoscopic resection of gastric GISTs[J].Surg Endosc,2017,doi:10.1007/s00464–017–5557–2.[Epub ahead of print]
[5]Otani Y,Furukawa T,Yoshida M,et al.Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases[J].Surgery,2006,139(4):484–492.
[6]Niimi K,Ishibashi R,Mitsui T,et al.Laparoscopic and endoscopic cooperative surgery for gastrointestinal tumor[J].Ann Transl Med,2017,5(8):187.doi:10.21037/atm.2017.03.35.
[7]Gotoda T,Ho KY,Soetikno R,et al.Gastric ESD:current status and future directions of devices and training [J].Gastrointest Endosc Clin N Am,2014,24(2):213–233.doi:10.1016/j.giec.2013.11.009.
[8]He L,Deng T,Luo H.Efficacy and safety of endoscopic resection therapies for rectal carcinoid tumors:a meta-analysis[J].Yonsei Med J,2015,56(1):72–81.doi:10.3349/ymj.2015.56.1.72.
[9]Facciorusso A,Antonino M,Di Maso M,et al.Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer:A meta-analysis[J].World J Gastrointest Endosc,2014,6(11):555–563.doi:10.4253/wjge.v6.i11.555.
[10]Lian J,Chen S,Zhang Y,et al.A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer[J].Gastrointest Endosc,2012,76(4):763–770.doi:10.1016/j.gie.2012.06.014.
[11]Jang JS,Choi SR,Graham DY,et al.Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions[J].Scand J Gastroenterol,2009,44(11):1370–1376.doi:10.3109/00365520903194609.
[12]Mannen K,Tsunada S,Hara M,et al.Risk factors for complications of endoscopic submucosal dissection in gastric tumors:analysis of 478 lesions[J].J Gastroenterol,2010,45(1):30–36.doi:10.1007/s00535–009–0137–4.
[13]Jeon SW,Jung MK,Cho CM,et al.Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions[J].Surg Endosc,2009,23(9):1974–1979.doi:10.1007/s00464–008–9988–7.
[14]Okada K,Yamamoto Y,Kasuga A,et al.Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm[J].Surg Endosc,2011,25(1):98–107.doi:10.1007/s00464–010–1137–4.
[15]Goto O,Fujishiro M,Kodashima S,et al.A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary:a retrospective analysis of postendoscopic submucosal dissection bleeding[J].Gastrointest Endosc,2010,71(2):241–248.doi:10.1016/j.gie.2009.08.030.
[16]Okano A,Hajiro K,Takakuwa H,et al.Predictors of bleeding after endoscopic mucosal resection of gastric tumors[J].Gastrointest Endosc,2003,57(6):687–690.
[17]Uedo N,Takeuchi Y,Yamada T,et al.Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer:a prospective randomized controlled trial[J].Am J Gastroenterol,2007,102(8):1610–1616.
[18]Takizawa K,Oda I,Gotoda T,et al.Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors[J].Endoscopy,2008,40(3):179–183.doi:10.1055/s–2007–995530.
[19]Oda I,Suzuki H,Nonaka S,et al.Complications of gastric endoscopic submucosal dissection[J].Dig Endosc,2013,25(Suppl 1):71–78.doi:10.1111/j.1443–1661.2012.01376.x.
[20]Toyokawa T,Inaba T,Omote S,et al.Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms:analysis of 1123 lesions[J].J Gastroenterol Hepatol,2012,27(5):907–912.doi:10.1111/j.1440–1746.2011.07039.x.
[21]Akasaka T,Nishida T,Tsutsui S,et al.Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm:multicenter survey by osaka university ESD study group[J].Dig Endosc,2011,23(1):73–77.doi:10.1111/j.1443–1661.2010.01062.x.
[22]Kakushima N,Fujishiro M.Endoscopic submucosal dissection for gastrointestinal neoplasms[J].World J Gastroenterol,2008,14(19):2962–2967.
[23]Kim ER,Kim JH,Kang KJ,et al.Is a second-look endoscopy necessary after endoscopic submucosal dissection for gastric neoplasm?[J].Gut Liver,2015,9(1):52–58.doi:10.5009/gnl13422.
[24]Park CH,Park JC,Lee H,et al.Second-look endoscopy after gastric endoscopic submucosal dissection for reducing delayed postoperative bleeding[J].Gut Liver,2015,9(1):43–51.doi:10.5009/gnl13252.
[25]Miyahara K,Iwakiri R,Shimoda R,et al.Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors:analysis of 1190 lesions in low- and high-volume centers in Saga,Japan[J].Digestion,2012,86(3):273–280.doi:10.1159/000341422.
[26]Yoon JY,Shim CN,Chung SH,et al.Impact of tumor location on clinical outcomes of gastric endoscopic submucosal dissection[J].World J Gastroenterol,2014,20(26):8631–8637.doi:10.3748/wjg.v20.i26.8631.
[27]Chung IK,Lee JH,Lee SH,et al.Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms:Korean ESD Study Group multicenter study[J].Gastrointest Endosc,2009,69(7):1228–1235.doi:10.1016/j.gie.2008.09.027.
[28]Oda I,Gotoda T,Hamanaka H,et al.Endoscopic submucosal dissection for early gastric cancer:Technical feasibility,operation time and complications from a large consecutive series[J].Digestive Endoscopy,2005,17(1):54–58.doi:10.1111/j.1443–1661.2005.00459.x.
[29]Takahashi F,Yoshitake N,Akima T,et al.A second-look endoscopy may not reduce the bleeding after endoscopic submucosal dissection for gastric epithelial neoplasm[J].BMC Gastroenterol,2014,14:152.doi:10.1186/1471–230X–14–152.
[30]Cho SJ,Choi IJ,Kim CG,et al.Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms[J].Endoscopy,2012,44(2):114–121.doi:10.1055/s–0031–1291459.
[31]Ojima T,Takifuji K,Nakamura M,et al.Complications of Endoscopic Submucosal Dissection for Gastric Noninvasive Neoplasia:An Analysis of 647 Lesions[J].Surg Laparosc Endosc Percutan Tech,2014,24(4):370–374.doi:10.1097/SLE.0b013e318290132e.
[32]Imagawa A,Okada H,Kawahara Y,et al.Endoscopic submucosal dissection for early gastric cancer:results and degrees of technical difficulty as well as success[J].Endoscopy,2006,38(10):987–990.
