主动脉夹层(aortic dissection,AD)是一种在主动脉中膜层出现退化,变性、坏死等病变基础上,内膜撕裂、血液经裂口注入主动脉壁,使中膜从外膜剥脱的一种病变。血管平滑肌细胞(vascular smooth muscle cells,VSMCs)是主动脉中膜层的主要成分,其增殖迁移能力的改变在AD形成中发挥着重要作用。Rho激酶(Rhoassociated coiled-coil containing protein kinase,ROCK)是最早发现的Rho下游效应分子,属于丝氨酸/苏氨酸蛋白激酶家族成员之一。ROCK与多种心血管疾病的发生有关,并且在血管平滑肌的收缩功能中扮演者重要角色。ROCK包括ROCK I和ROCK II两种亚型,两者的氨基酸序列具有65%的同源性,其中激酶区的同源性高达90%[1]。在大鼠平滑肌细胞的迁移中,ROCK I起主导作用,而ROCK II的作用不明显;在大鼠平滑肌细胞的增殖中,两者的作用均不明显[2]。近期有研究[3]显示,转化生长因子β1(TGF-β1)可呈浓度依赖性诱导人主动脉平滑肌细胞的增殖和迁移,从而促使AD的发生。本研究通过下调VSMC的ROCK I和ROCK II基因,观察其在TGF-β1刺激下对人主动脉平滑肌细胞迁移和增殖的影响。
1 材料与方法
1.1 材料
细胞:人主动脉平滑肌细胞(H AVSMCs),购于美国ATCC公司。主要试剂:TGF-β1(美国Peprotech公司);胎牛血清(杭州天杭生物科技有限公司);胰酶-EDTA(吉诺生物医药技术有限公司);DMEM高糖培养基(HyClone);PBS(吉诺生物医药技术有限公司);青霉素-链霉素溶液(100×)(吉诺生物医药技术有限公司);siRNA(广州锐博);Y-27632(美国Selleck);Opti-MEM I Reduced serum medium(美国Gibco);Lipofectamine 2000 Transfection Reagent(美国Invitrogen);GAPDH(ab37168)、ROCK I(ab45171)以及ROCK II(ab125025)(abcam公司);HRP-Goat anti Rabbit(074-1506)(KPL);GNM14170型D-Hanks(吉诺生物医药技术有限公司);AS1086型结晶紫染液(武汉阿斯本生物技术有限公司);CCK-8检测试剂盒(C0038)(碧云天生物技术有限公司)。主要仪器:SCO6WE型CO2恒温培养箱(SHEL LAB);SW-CJ-1FD型洁净工作台(苏净安泰);IX51型倒置显微镜(OLYMPUS);L400型离心机(湖南湘仪实验室仪器开发有限公司);3422型Transwell板(Corning);DR-200Bs型酶标检测仪(Diatek)。
1.2 实验方法
1.2.1 细胞培养 在37 ℃、5%CO2条件下用含10%FBS的高塘DMEM培养基培养 HA-VSMCs。培养基按照1:100加入青链霉素混合液(含1 000 U/mL青霉素和10 mg/mL链霉素)。HA-VSMCs培养达85%~90%融合率时用胰酶消化细胞,用细胞计数板计数,稀释至所需浓度,按照需要进行细胞干预及后续实验。
1.2.2 siRNA 转染 ⑴ 转染前1 d,胰酶消化收集细胞,加入不含抗生素的培养基,调整细胞密度为2×105个/mL,以每孔2 mL接种至6孔板。转染时要求细胞汇合度为50%~60%。⑵ 转染液制备(siRNA储存液浓度为20 μM),每孔用量如下:用 190 μL Opti-MEI无血清培养基将 10 μL siRNA稀释,轻轻混匀。使用前轻轻摇匀Lipofectamine 2000,取 20 μL Lipofectamine 2000 在 18 μL 无血清培养基中稀释,室温孵育5 min。将前2步缩稀释的siRNA和Lipofectamine 2000混合(总体积400 μL),轻轻混匀,室温静置20 min。⑶ 将孔板内的培养基更换为不含FBS的DMEM培养基,转染孔加1.6 mL,空白孔加2 mL。⑷ 在需要转染的孔中加入400 μL转染液,轻轻摇匀。⑸ 37 ℃培养,转染4~6 h后,细胞换液为含FBS的DMEM培养基,24 h后倒置显微镜下观察转染效果,记录图片,48 h后可检测蛋白表达。
1.2.3 细胞干预实验分组和药物干预(一) ⑴ 空白对照组:正常细胞在完全培养基中培养24 h;⑵ ROCK I siRNA组:ROCK I siRNA转染成功的细胞在完全培养基中培养24 h;⑶ ROCK II siRNA组:ROCK II siRNA转染成功的细胞在完全培养基中培养24 h;⑷ TGF-β1组:正常细胞在含5 ng/mL TGF-β1的完全培养基中培养24 h;⑸ ROCK I siRNA+TGF-β1组: 转 染 ROCK I siRNA的细胞在含5 ng/mL TGF-β1的完全培养基中培养24 h;⑹ ROCK II siRNA+TGF-β1组:转染ROCK II siRNA的细胞在含5 ng/mL TGF-β1的完全培养基中培养24 h。
1.2.4 Western blot检测 上述细胞培养及干预处理后,PBS洗2~3次,加入适量RIPA裂解液,收集细胞至EP管中,4 ℃,12 000 r/min离心10 min;使用BCA蛋白质浓度测定试剂盒测定样品蛋白浓度;根据蛋白分子量配制不同浓度SDS-PAGE,样品蛋白上样量为40 μg/孔,电泳条件为浓缩胶80 V,分离胶120 V,至溴酚蓝到达胶板下沿,300 mA电流60 min将蛋白转至PVDF膜;将转好的膜加入封闭液室温摇床封闭1 h。除去封闭液,加入用封闭液稀释好的ROCK I和ROCK II抗体4 ℃过夜;TBST充分洗涤PVDF膜3次,5 min/次,加入用封闭液稀释好的二抗,室温摇床孵育30 min;再用TBST充分洗涤PVDF膜3次,5 min/次,加入适量ECL底物,X光胶片压片后放入显影液显影、定影液定影;使用AlphaEaseFC软件处理系统分析目标带的灰度值,将目的灰度除以内参的灰度,以比较各组目的蛋白的相对表达量。
1.2.5 细胞干预实验分组和药物干预(二) ⑴ 空白对照组:正常细胞在完全培养基中培养24 h;⑵TGF-β1组:正常细胞在含5 ng/mL TGF-β1的完全培养基中培养24 h;⑶ ROCK I siRNA组:转染Rock I siRNA的细胞在含5 ng/mL TGF-β1的完全培养基中培养24 h;⑷ ROCK II siRNA组:转染ROCK II siRNA的细胞在含5 ng/mL TGF-β1的完全培养基中培养24 h;⑸ Y-27632组:Y-27632是ROCK I选择性抑制剂,正常细胞用10 μM Y-27632预处理1 h,之后换培养液,在含5 ng/mL TGF-β1的完全培养基中培养24 h。
1.2.6 细胞迁移实验(Transwell试验) ⑴ 配制染液:0.5%的结晶紫溶液,用时用PBS溶液1:5稀释成0.01%的结晶紫染液;⑵ 将细胞制成105个/mL悬液,取1 mL细胞悬液于1 500 r/min离心5 min,弃上清;⑶ 加入1 mL无血清培养基,吹打均匀后取200 μL细胞悬液放入Transwell小室中;⑷ 24孔板中加入500 μL含10%FBS的完全培养基,将小室放入板中;⑸ 37℃下于CO2 (含量5%)培养箱中培养48 h;⑹ 染色:将小室取出,用PBS洗去培养基,用棉签将上室中的胶和细胞擦除干净,结晶紫染色10 min,洗去表面的结晶紫,于倒置显微镜下对非细胞接种侧拍照。
1.2.7 细胞增殖试验(CCK-8试验) ⑴ 将对数生长期细胞用胰蛋白酶消化,配制成浓度为1×105个/mL的细胞悬液,按10 000细胞/孔接种于96孔板,每孔加100 μL,置于CO2(5%)培养箱中37℃下培养24 h以贴壁;⑵ 继续培养24 h分别更换为100 μL细胞样品各自对应的含有一定浓度药物的培养基,对照组更换为含溶剂的培养基,每个浓度的样本设5个重复;⑶ 所有孔中加入10 μL CCK-8溶液,培养箱中孵育1~4 h;⑷ 使用酶标仪测定450 nm光吸收值,以溶剂处理的细胞为对照组,不含细胞的培养基为空白组。
1.3 统计学处理
结果用SPSS软件做方差分析,统计数据用均值±标准差(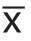 ±s)表示,P<0.05为差异有统计学意义。
±s)表示,P<0.05为差异有统计学意义。
2 结 果
2.1 转染效果鉴定及不同处理后ROCK I与ROCK II蛋白表达
对HA-VSMCs进行ROCK I siRNA和 ROCK II siRNA 转染24 h后使用倒置显微镜观察转染结果,镜下可见大量平滑肌细胞转染成功(图1)。转染后48 h,Western blot检测转染后ROCK I和ROCK II蛋白表达水平。与空白对照组比较,ROCK I siRNA组ROCK I蛋白表达减少,TGF-β1组ROCK I蛋白表达增加(均P<0.05);与TGF-β1组相比,ROCK I siRNA+TGF-β1组ROCK I蛋白表达减少(P<0.05),ROCK II siRNA+TGF-β1组对ROCK I蛋白表达无明显影响(P>0.05)。与空白对照组比较,ROCK I siRNA组ROCK II蛋白表达减少(P<0.05),而TGF-β1组ROCK II蛋白表达无明显变化(P>0.05);与TGF-β1组比较,ROCK I siRNA+TGF-β1组ROCK II蛋白表达无明显变化(P>0.05),ROCK II siRNA+TGF-β1组ROCK II蛋白表达减少(P<0.05)(图2)。
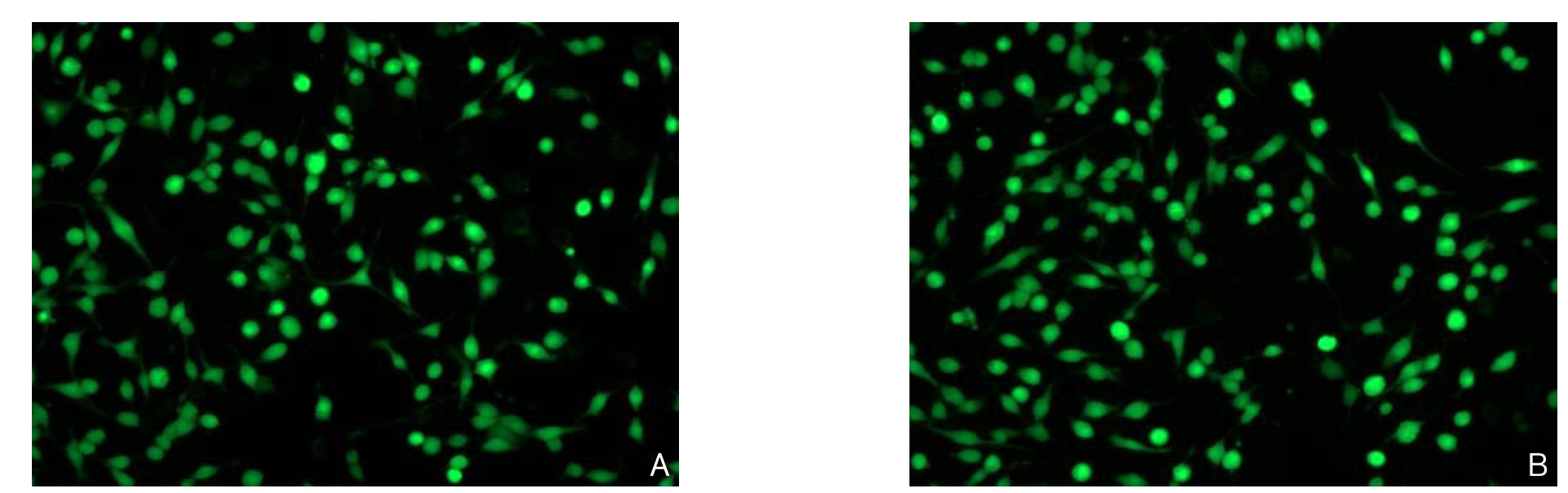
图1 荧光转染结果(×100) A:ROCK I siRNA转染;B:ROCK II siRNA转染
Figure 1 Fluorescence transfection results (×100) A: ROCK I siRNA transfection; B:ROCK II siRNA transfection
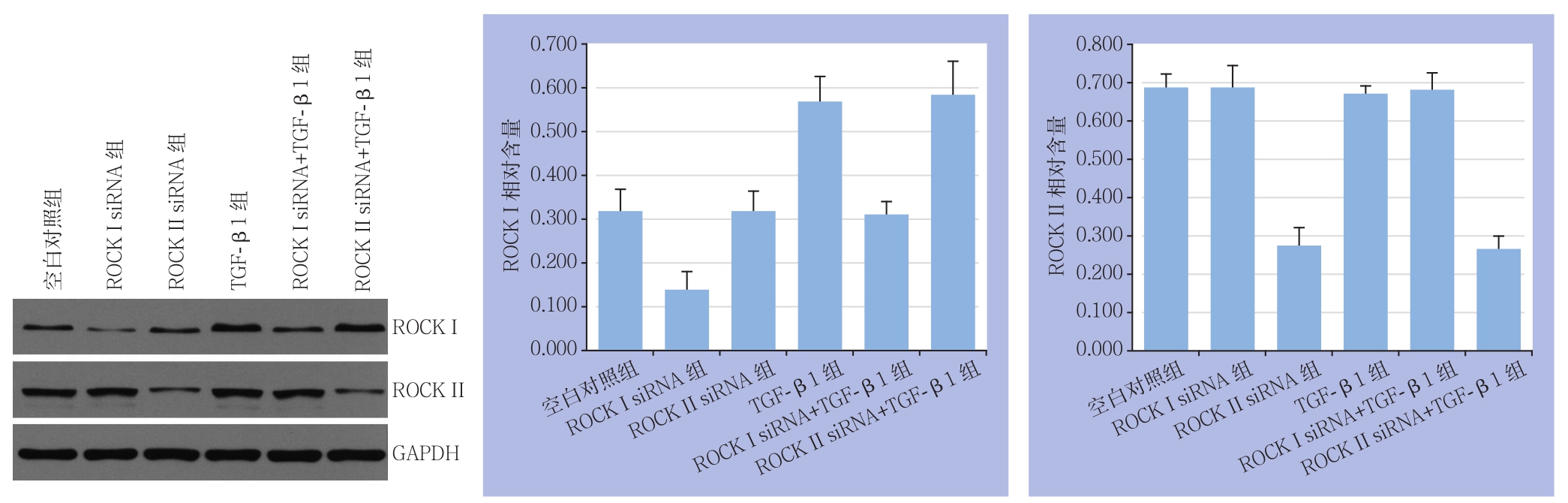
图2 Western bolt检测ROCK I和ROCK II蛋白的表达
Figure 2 Western blot analysis of ROCK I and ROCK II protein expressions
2.2 ROCK I和ROCK II对TGF-β1诱导的HAVSMCs迁移作用的影响
与空白对照组相比,TGF-β1组HA-VSMCs迁移细胞数增加[(208.3±18.9 )vs. (23.8±1.5),P<0.0 5];与T G F-β 1组相比,R O C K I siRNA+TGF-β1组、Y-27632+TGF-β1组细胞迁移数减少[(61.0±1.8) vs. (208.3±18.9);(63.3±7.4) vs. (208.3±18.9),均P<0.05],而ROCK II siRNA+TGF-β1组对细胞迁移无明显变化(P>0.05);与Y-27632+TGF-β1组比较,ROCK I siRNA+TGF-β1组细胞迁移数无统计学差异[(61.0±1.8) vs. (63.3±7.4),P>0.05],而ROCK II siRNA+TGF-β1组细胞迁移数增加[(274.5±10.0 )vs.( 63.3±7.4),P<0.05]。对迁移后Transwell小室进行结晶紫染色,倒置显微镜下观察,TGF-β1组和ROCK II siRNA+TGF-β1组平滑肌细胞数量较多;C o n t r o l组、R O C K I siRNA+TGF-β1组和Y-27632+TGF-β1组平滑肌细胞数量较少(图3)。
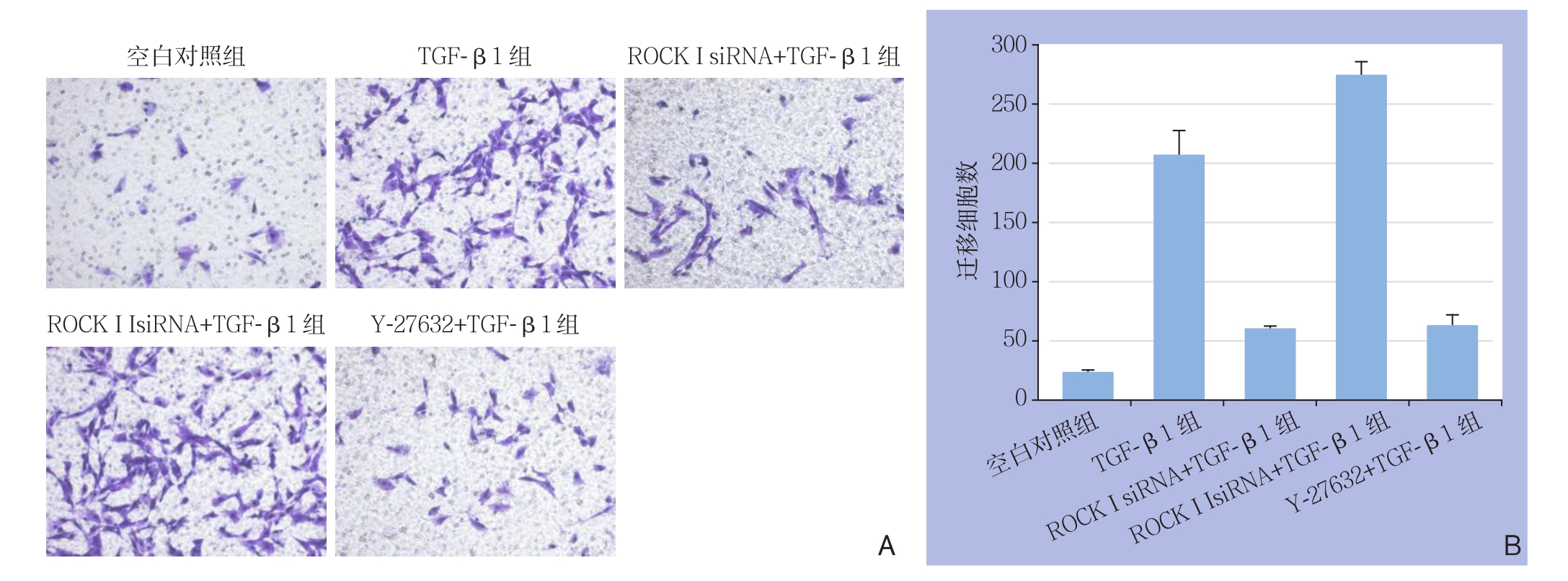
图3 细胞迁移实验 A:结晶紫染色法观察(×100);B:各组迁移细胞数比较Figure 3 Cell migration assay A: Crystal violet staining observation (×100); B: Comparison of the numbers of migrating cells among groups
2.3 ROCK I和ROCK II对TGF-β1诱导的HAVSMCs增殖的影响
与空白对照组相比,TGF-β1组OD值升高[(1.350±0.057) vs. (252±0.061),P<0.05];与TGF-β1组比较,ROCK I siRNA+TGF-β1组、ROCK II siRNA+TGF-β1组、Y-27632+TGF-β1组OD值均无统计学差异(均P>0.05)(图4)。
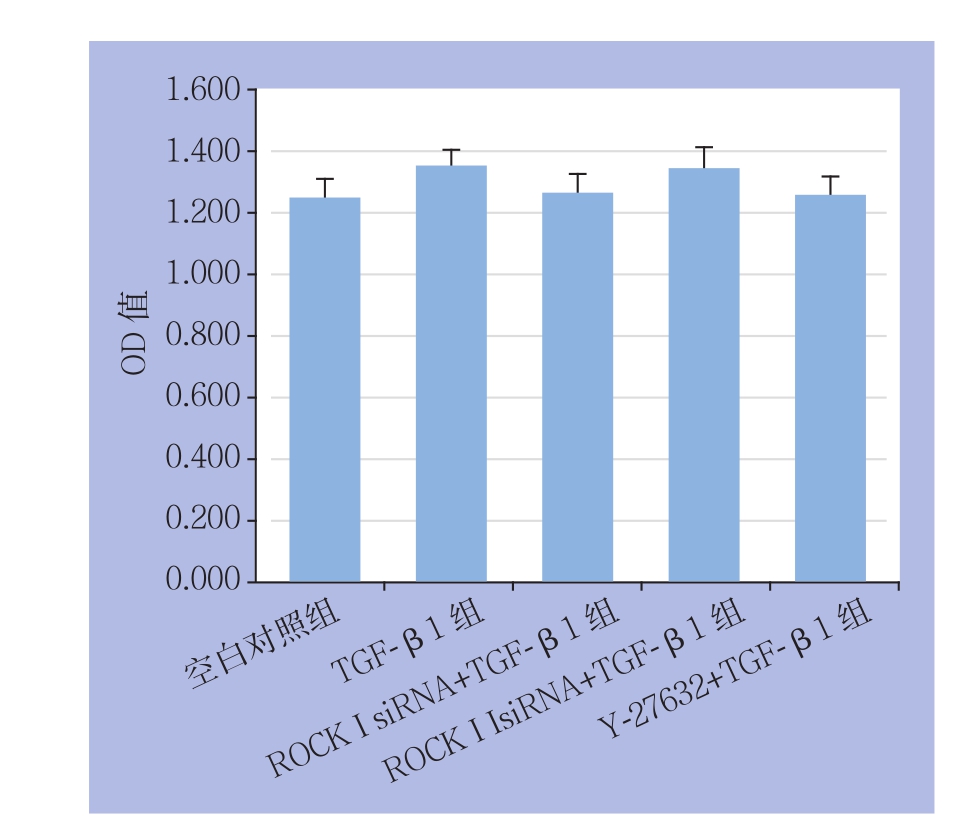
图4 各组对HA-VSMCs增殖情况比较
Figure 4 Comparison of proliferations of among each group of HA-VSMCs
3 讨 论
近年来,随着生活水平的提高,AD的发生率呈逐年增加的趋势。目前,有关主动脉夹层的回顾性研究分析证实,胸主动脉腔内修复术(thoracic endovascular aortic repair,TEVAR)是治疗AD的首选治疗方案[4-9]。AD常表现为突然地剧烈撕裂样或剥脱样疼痛,其起病急、进展快且病死率高[10]。因此,有关AD的相关基础研究也成为了目前热点课题。
目前研究表明,AD的起始病变位于主动脉中膜层VSMC。在病理刺激下,VSMC的异常增殖与迁移可导致血管结构重构等一系列病理变化。Rho蛋白家族是一类小GTP结合蛋白,目前研究最多是Rho、Rac和Cdc42 3个亚家族,可通过下游效应分子的活性参与细胞骨架动力学。ROCK是首个被发现的小G蛋白下游效应分子,与Rho蛋白一起调节细胞肌动蛋白,与细胞的增殖迁移等多种生物功能密切相关[11]。ROCK在缺氧环境下促进VSMC的增殖与迁移,且可通过促进MMP-2表达从而促使VSMC增殖[12-13]。ROCK包括ROCK I和ROCK II两种亚型。有研究通过siRNA转染下调ROCK I和ROCK II基因表达证实,ROCK I在大鼠平滑肌细胞的迁移中起主导作用,而ROCK II的作用不明显;在大鼠平滑肌细胞的增殖中,两者的作用均不明显。同时又有人通过过表达ROCK I基因证实,ROCK I在大鼠主动脉血管平滑肌细胞的迁移中起主导作用[14];通过自行设计的针对ROCK II的siRNA转染血管平滑肌细胞使其ROCK II基因下调后观察细胞迁移数量并未受到影响[15]。在AD患者病变组织的中膜层中发现TGF-β1表达明显增强,同时有研究[16-18]显示TGF-β1可呈浓度依赖性诱导HA-VSMCs发生增殖与迁移,而VSMC是主动脉中膜的主要成分。因此,TGF-β1在体内的表达增加诱导HA-VSMCs的增殖与迁移可能是促使主动脉夹层形成的一个重要因素。
为探讨R OCK I和ROC K I I对TGF-β1诱导的HA-VSMCs迁移增殖的影响。本研究利用siRNA技术,使HA-VSMCs的ROCK I和ROCK II基因表达下调,并分别对比加入TGF-β1组和未加入TGF-β1组的ROCK I和ROCK II蛋白表达,结果发现TGF-β1对转染后的蛋白表达有显著影响。又通过Transwell试验和CCK-8试验检测ROCK两种亚型对TGF-β1诱导的HA-VSMCs迁移和增殖的影响。结果显示,与TGF-β1组相比,ROCK I siRNA+TGF-β1组和Y-27632+TGF-β1组对H A-V S M C s迁移起抑制作用,R O C K I I siRNA+TGF-β1组则无明显作用。与TGF-β1组相比,ROCK I siRNA、ROCK II siRNA、Y-27632对HA-VSMCs增殖均没有明显影响。由此可见,ROCK I在TGF-β1诱导的HA-VSMCs迁移中起重要作用,而ROCK II的作用不明显;ROCK I和ROCK II对TGF-β1诱导的HA-VSMCs增殖的影响无统计学差异。
在AD患者中膜层平滑肌细胞的迁移、增殖能力明显增强,发生了由收缩型向合成型的表型转化[19]。有研究[20]显示,TGF-β1可诱导HA-VSMCs发生表型转化,其收缩型的标志蛋白α-平滑肌肌动蛋白(smooth muscle α-actin,α-SMA)、平滑肌22α(smooth muscle 22α,SM22α)减少,合成型的标志蛋白骨桥蛋白(osteopontin,OPN)增加。因此,下一步将进一步探讨ROCK I和ROCK II对TGF-β1诱导的HA-VSMCs表型转化的影响,对主动脉夹层的机制进行进一步的探索。
参考文献
[1]Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system[J]. Am J Physiol Cell Physiol, 2006,290(3):C661–668.
[2]赵莹, 杨福春, 魏晓晴, 等. ROCK I/II基因下调对血管平滑肌细胞迁移及增殖的影响[J]. 现代生物医学进展, 2009, 9(17):3232–3234.Zhao Y, Yang FC, Wei ZQ, et al. Effects of ROCK I/II gene expression down-regulated on migration and proliferation of vascular smooth muscle cells[J]. Progress in Modern Biomedicine,2009, 9(17):3232–3234.
[3]朱水波, 周孜孜, 郗二平, 等. 不同浓度转化生长因子β1对人主动脉平滑肌细胞增殖及迁移的影响[J]. 临床外科杂志, 2016,24(5):366–368. doi:10.3969/j.issn.1005–6483.2016.05.016.Zhu SB, Zhou ZZ, Xi EP, et al. Effects of TGF-β1 on proliferation and migration of human aortic vascular smooth muscle cells[J].Journal of Clinical Surgery, 2016, 24(5):366–368. doi:10.3969/j.issn.1005–6483.2016.05.016.
[4]朱水波, 朱健, 郗二平, 等. 不开胸“杂交”手术治疗主动脉弓部夹层的近中期随访研究[J]. 中国胸心血管外科临床杂志, 2016,23(10): 988–991. doi: 10.7507/1007–4848.20160236.Zhu SB, Zhu J, Xi EP, et al. Hybrid Procedure without Sternotomy for Aortic Arch Dissection: A Short- and Mid-term Follow-up[J].Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2016, 23(10):988–991. doi: 10.7507/1007–4848.20160236.
[5]张瑜, 朱健, 朱水波, 等. 累及弓部主动脉夹层手术方式选择及疗效[J]. 中国普通外科杂志, 2016, 25(6):823–827. doi:10.3978/j.issn.1005–6947.2016.06.007.Zhang Y, Zhu nJ, Zhu SB, et al. Selection of surgical procedures and efficacy analysis in treatment of aortic dissection involving aortic arch[J]. Chinese Journal of General Surgery, 2016, 25(6):823–827.doi:10.3978/j.issn.1005–6947.2016.06.007.
[6]朱健. 腔内修复术救治胸主动脉创伤的动物实验与临床研究[D].广州: 南方医科大学, 2013:85.Zhu J. Endovascular aortic repair rescuing thoracic aortic trauma:animal experimental and clinical studying[D]. Guangzhou: Southern Medical University2013:85.
[7]郗二平, 朱健, 朱水波, 等. 腔内修复术治疗急慢性Debakey III型夹层的临床研究[J]. 中华全科医学, 2013, 11(5): 686–688.Xi EP, Zhu J, Zhu SB, et al. The Clinical Studying of Endovascular Aortic Repair for Acute and Chronic Aortic Dissection[J]. Chinese Journal of General Practice, 2013, 11(5): 686–688.
[8]朱水波, 朱健, 郗二平, 等. 胸主动脉腔内修复术治疗复杂性胸主动脉夹层的临床疗效[J]. 中国循环杂志, 2016, 31(8):789–792.doi:10.3969/j.issn.1000–3614.2016.08.015.Zhu SB, Zhu J, Xi EP, et al. Clinical Efficacy of Thoracic Endovascular Aortic Repair for Treating the Patients With Complicated Thoracic Aortic Dissection[J]. Chinese Circulation Journal, 2016, 31(8):789–792. doi:10.3969/j.issn.1000–3614.2016.08.015.
[9]朱健, 郗二平, 朱水波, 等. 主动脉腔内修复术救治胸主动脉破裂的临床观察[J]. 中国普通外科杂志, 2016, 25(12):1689–1693.doi:10.3978/j.issn.1005–6947.2016.12.004.Zhu J, Xi EP, Zhu SB, et al. Clinical observation of thoracic endovascular aortic repair for emergency treatment of thoracic aortic rupture[J]. Chinese Journal of General Surgery, 2016,25(12):1689–1693. doi:10.3978/j.issn.1005–6947.2016.12.004.
[10]Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study[J]. Circulation, 2013, 127(20):2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483.
[11]Schofield AV, Bernard O. Rho-associated coiled-coil kinase (ROCK)signaling and disease[J]. Crit Rev Biochem Mol Biol, 2013,48(4):301–316. doi: 10.3109/10409238.2013.786671.
[12]Walker J, Undem C, Yun X, et al. Role of Rho kinase and Na+/H+exchange in hypoxia-induced pulmonary arterial smooth muscle cell proliferation and migration[J]. Physiol Rep, 2016, 4(6):pii:e12702.doi: 10.14814/phy2.12702.
[13]Cui Y, Sun YW, Lin HS, et al. Platelet-derived growth factor-BB induces matrix metalloproteinase-2 expression and rat vascular smooth muscle cell migration via ROCK and ERK/p38 MAPK pathways[J]. Mol Cell Biochem, 2014, 393(1/2):255–263. doi:10.1007/s11010–014–2068–5.
[14]林海双, 赵莹, 高颖. ROCK对PDGF介导血管平滑肌细胞迁移分子机制的研究[C]//泛环渤海地区九省市生物化学与分子生物学会——2011年学术交流会论文集. 太原: 泛环渤海地区九省市生物化学与分子生物学会——2011年学术交流会委员会,2011:116–117.Lin SH, Zhao Y, Gao Y. Mechanism of ROCK in PDGF mediated migration of vascular smooth muscle cells[C]//Society of Biochemistry and Molecular Biology of Nine Provinces in Pan-Bohai Sea Region——Proceedings of the 2011 Academic Symposium. Taiyuan: Society of Biochemistry and Molecular Biology of Nine Provinces in Pan-Bohai Sea Region——Committee of the 2011 Academic Symposium, 2011:116–117.
[15]张金超. ROCK-II基因表达下调与血管平滑肌细胞迁移的关系[D]. 大连:大连医科大学, 2008:1–48.Zhang JC. Down-regulation of ROCK-II and the migration of vascular smooth muscle cell[D]. Dalian: Dalian Medical University,2008:1–48.
[16]Grond-Ginsbach C, Pjontek R, Aksay SS, et al. Spontaneous arterial dissection: phenotype and molecular pathogenesis[J]. Cell Mol Life Sci, 2010, 67(11):1799–1815. doi: 10.1007/s00018–010–0276–z.
[17]Wang X, LeMaire SA, Chen L, et al. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection[J]. Circulation, 2006, 114(1 Suppl):I200–205.
[18]杨守国, 王春生, 陈昊, 等. 胸主动脉夹层动脉壁TGF-β1表达与细胞外基质分布[J]. 中华胸心血管外科杂志, 2010, 26(1):33–36.doi:10.3760/cma.j.issn.1001–4497.2010.01.013.Yang SG, Wang CS, Chen H, et al. TGF-β1 expression and distribution in the extracellular matrix of the dissected wall of thoracic aorta[J]. Chinese Journal of Thoracic and Cardiovascular Surgery, 2010, 26(1):33–36. doi:10.3760/cma.j.issn.1001–4497.2010.01.013.
[19]Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease[J]. Annu Rev Physiol, 2012, 74:13–40.doi: 10.1146/annurev-physiol–012110–142315.
[20]周孜孜, 郗二平, 王荣平, 等. 转化生长因子-β1对人主动脉平滑肌细胞表型转化的影响及其机制[J]. 中华实验外科杂志, 2015,32(11):2771–2774. doi:10.3760/cma.j.issn.1001–9030.2015.11.043.Zhou ZZ, Xi EP, Wang RP, et al. Effect of transforming growth factor-β1 on human aortic vascular smooth muscle cell phenotype switch and mechanism[J]. Chinese Journal of Experimental Surgery, 2015, 32(11):2771–2774. doi:10.3760/cma.j.issn.1001–9030.2015.11.043.
