原发性肝癌(以下简称肝癌)患者行肝癌R0切除出现术后肝衰竭(posthepatectomy liver failure,PHLF)严重影响患者的预后[1],因此术前肝功能评估尤为重要。Child-Pugh(CP)分级标准是临床上最常用来评价肝功能的指标,终末期肝病模型(model for end-stage liver disease,MELD)的确定基于大量统计学数据[2],常被用于评价失代偿期肝脏疾病的严重程度[3-4],2015年有学者[5]基于大样本的Cox多因素回归分析,提出了白蛋白-胆红素(albumin-bilirubin,ALBI)评分模型,将肝病患者分为3个风险类别。本文回顾性分析我院2010年9月—2015年11月275例肝癌患者,借助ROC曲线比较3种预测模型的准确性,以期在肝癌患者的手术治疗前做出更优的选择,减少患者围手术期死亡。
1 资料与方法
1.1 研究对象
2010年9月—2015年11月所有在南昌大学第一附属医院接受肝癌R0切除术的肝癌患者均纳入该回顾性研究。纳入标准为:首次肝癌R0切除;肝切除术前未进行其他肿瘤相关治疗;未并发其他恶性肿瘤;肝切除术前无心肺、肾或脑功能障碍。
1.2 方法
1.2.1 定义 肝切除术前肿瘤状态可由CT或MRI进行评估。肝癌及肝硬化的诊断基于术后病理报告。肝癌分期根据肝癌TNM分期进行。CP得分用于评估术前肝功能。R0切除[6]定义为完整切除所有可见的肿瘤,且手术切除标本的病理检查显示手术切缘中无残留癌细胞。临床显著门脉 高 压(clinically significant portal hypertension,CSPH)定义为内镜下胃食管静脉曲张或脾肿大(直径>12 cm)伴血小板计数低于100×109 g/L[7]。定义大范围肝切除为切除至少3个Couinaud肝段[8],小范围肝切除为切除1~2个Couinaud肝段[9]。PHLF的定义方式较多,其中国际肝脏外科学组(International Study Group of Liver Surgery,ISGLS)[10]的定义和法国Balzan等[11]报告的50-50标准(50-50 criteria)在国内外较常应用且经过大量临床病例验证[12-14]。前者经文献检索及临床检验于2011年提出[10],定义为“肝切除术后肝脏出现获得性损伤,无法维持其正常的合成、分泌及解毒功能,其临床表现为术后第5天或其后出现高胆红素血症及INR升高”;后者通过纳入775例肝切除患者,借助统计学方法(Logistic多因素回归模型)得出[11],定义为“术后第5天,凝血酶原时间活动度(prothrombin time,PT)<50%且血清胆红素(serum bilirubin,SB)>50 μmol/L”。
1.2.2 随访 所有患者均通过门诊或电话进行定期随访,术后前3个月每月随访1次,3个月后首年每隔2个月随访1次,术后1年之后每3个月随访1次。每次随访收集临床、实验室、影像学资料,包括血清甲胎蛋白水平、血清生化、胸片、腹部超声、腹部CT或MRI等。
1.2.3 分值计算 ⑴ CP值依据总胆红素、白蛋白、凝血酶原时间,肝性脑病及腹水的临床表现进行计算。CP分级定义如下:A级5~6分;B级7~9分;C级10~15分[15]。⑵ MELD值根据以下公式计算:R=0.378ln[胆红素(mg/dL)]+1.12ln(INR)+0.95ln[肌酐(mg/dL)]+0.64(病因:胆汁性或酒精性0,其他1)。R值越高,其风险越大,生存率越低[16]。⑶ ALBI值根据以下公式计算:0.66×log10{总胆红素(μmol/L)-0.085[白蛋白](g/L)}。ALBI分级定义如下:1级(低于-2.60),2级(-2.59~-1.39),3级(高于 -1.39) [5]。
1.3 统计学处理
应用Medcalc 15.0软件进行统计分析。平均值±标准差( ±s)用于描述正态分布资料,中位数(四分位数)[M(Qn)]用于描述偏态分布资料,数量和构成比[n(%)]用于描述分类变量。依据ISGLS和50-50两种诊断标准分组,每组再依据Couinaud肝段切除数目分为大范围切除组和小范围切除组两个亚组进行分析,以排除肝脏切除体积对结果的影响。计算受试者工作特征曲线下面积(areas under the receiver operating characteristic curve,AUROC)及95%置信区间(95% CI)用于评估3种评分的预测能力,应用De-long检验和Z检验比较3者AUROC,检验水准α=0.05。同时计算每种评分的截断值,灵敏度,特异度,阳性预测值(positive predictive value,PPV),阴性预测值(negative predictive value,NPV),阳性似然比(positive likelihood ratio,PLR),阴性似然比(negative likelihood ratio,NLR)。
±s)用于描述正态分布资料,中位数(四分位数)[M(Qn)]用于描述偏态分布资料,数量和构成比[n(%)]用于描述分类变量。依据ISGLS和50-50两种诊断标准分组,每组再依据Couinaud肝段切除数目分为大范围切除组和小范围切除组两个亚组进行分析,以排除肝脏切除体积对结果的影响。计算受试者工作特征曲线下面积(areas under the receiver operating characteristic curve,AUROC)及95%置信区间(95% CI)用于评估3种评分的预测能力,应用De-long检验和Z检验比较3者AUROC,检验水准α=0.05。同时计算每种评分的截断值,灵敏度,特异度,阳性预测值(positive predictive value,PPV),阴性预测值(negative predictive value,NPV),阳性似然比(positive likelihood ratio,PLR),阴性似然比(negative likelihood ratio,NLR)。
2 结 果
2.1 总体样本基线特征
2010年9月—2015年11月共计336例肝癌患者接受肝癌R0切除术,其中61例(18.2%)因以下原因被排除在外:36例(10.7%)在术前接受其他癌症相关治疗,14例(4.2%)并发其他恶性肿瘤,11例(3.3%)术前存在心肺、肾或脑功能障碍,剩余275例均纳入本研究中。
275例患者的平均年龄为51.8岁,大部分为男性(74.5%),乙肝阳性率高(86.5%),且近一半肝癌患者同时伴有肝硬化(49.5%),多不伴有腹水(72.0%)。肿瘤平均大小为6.55 cm,且多为早期单发性中高分化瘤,故TNM分期I期占多数(80.7%),高失血量者较多(47.6%),可能与切除巨块型肿瘤有关。小范围切除组共203例(73.8),大范围切除组共72例(26.2%)(表1)。3种评分得分及其等级显示,大部分患者为CP A级(85.5%),B级患者占14.5%,无C级患者;ALBI评分中仅7例患者为3级(2.5%),剩余患者多为1级(60.7%)。CP、MELD、ALBI的平均分为5.61、-2.66、10.862(表2)。
表1 275例患者的基线特征
Table 1 Baseline characteristics of the 275 patients
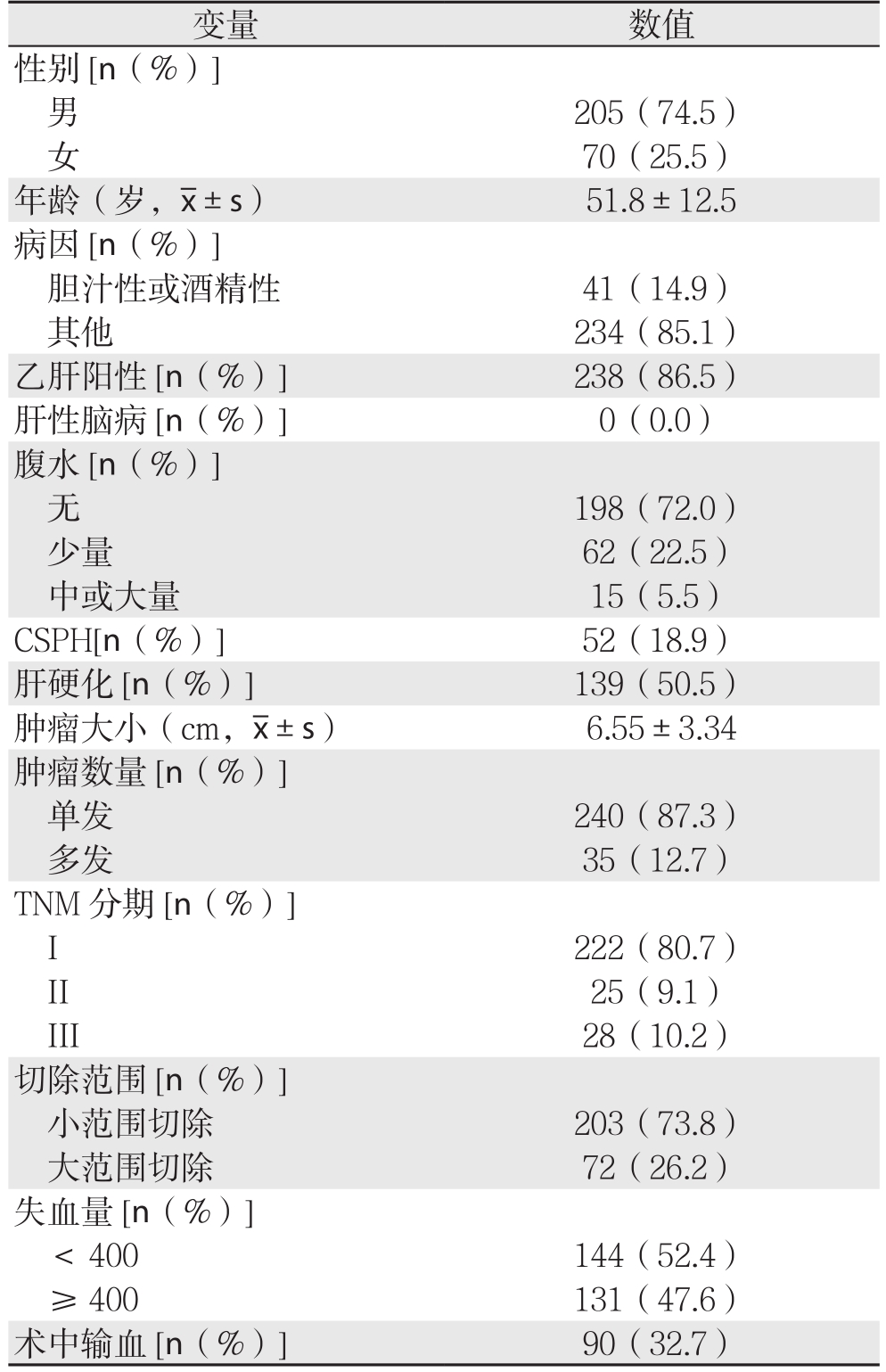
变量 数值性别[n(%)]男205(74.5)女70(25.5)年龄(岁, ±s) 51.8±12.5病因[n(%)]胆汁性或酒精性 41(14.9)其他 234(85.1)乙肝阳性[n(%)] 238(86.5)肝性脑病[n(%)] 0(0.0)腹水[n(%)]无198(72.0)少量 62(22.5)中或大量 15(5.5)CSPH[n(%)] 52(18.9)肝硬化[n(%)] 139(50.5)肿瘤大小(cm,
±s) 51.8±12.5病因[n(%)]胆汁性或酒精性 41(14.9)其他 234(85.1)乙肝阳性[n(%)] 238(86.5)肝性脑病[n(%)] 0(0.0)腹水[n(%)]无198(72.0)少量 62(22.5)中或大量 15(5.5)CSPH[n(%)] 52(18.9)肝硬化[n(%)] 139(50.5)肿瘤大小(cm, ±s) 6.55±3.34肿瘤数量[n(%)]单发 240(87.3)多发 35(12.7)TNM分期[n(%)]I 222(80.7)II 25(9.1)III 28(10.2)切除范围[n(%)]小范围切除 203(73.8)大范围切除 72(26.2)失血量[n(%)]< 400 144(52.4)≥ 400 131(47.6)术中输血[n(%)] 90(32.7)
±s) 6.55±3.34肿瘤数量[n(%)]单发 240(87.3)多发 35(12.7)TNM分期[n(%)]I 222(80.7)II 25(9.1)III 28(10.2)切除范围[n(%)]小范围切除 203(73.8)大范围切除 72(26.2)失血量[n(%)]< 400 144(52.4)≥ 400 131(47.6)术中输血[n(%)] 90(32.7)
表2 3种评分预测275例患者得分及等级
Table 2 Scores and grades of the 275 patients obtained by three scoring systems
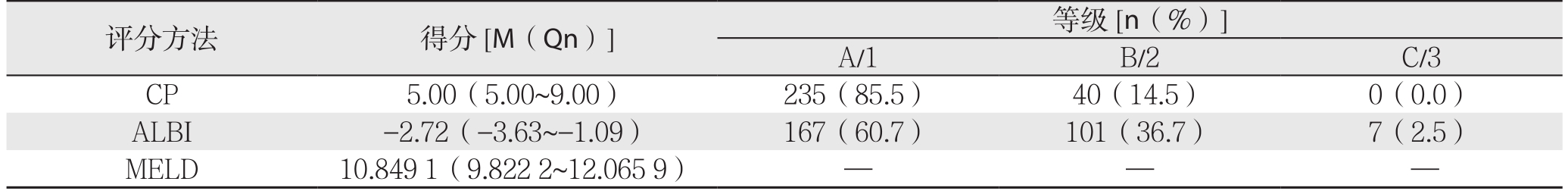
评分方法 得分[M(Qn)] 等级[n(%)]A/1 B/2 C/3 CP 5.00(5.00~9.00) 235(85.5) 40(14.5) 0(0.0)ALBI -2.72(-3.63~-1.09) 167(60.7) 101(36.7) 7(2.5)MELD 10.8491(9.8222~12.0659) — — —
2.2 50-50标准下3种评分预测PHLF
50-50标准下PHLF诊断结果显示,共43例被诊断为术后出现肝功能衰竭,其中大范围切除组中检出14例,小范围切除组中检出29例(表3)。
表3 3种评分基于50-50和ISGLS标准预测275例患者PHLF结果
Table 3 Results of the three scoring systems for predicting PHLF in the 275 patients according to 50-50 and ISGLS criteria
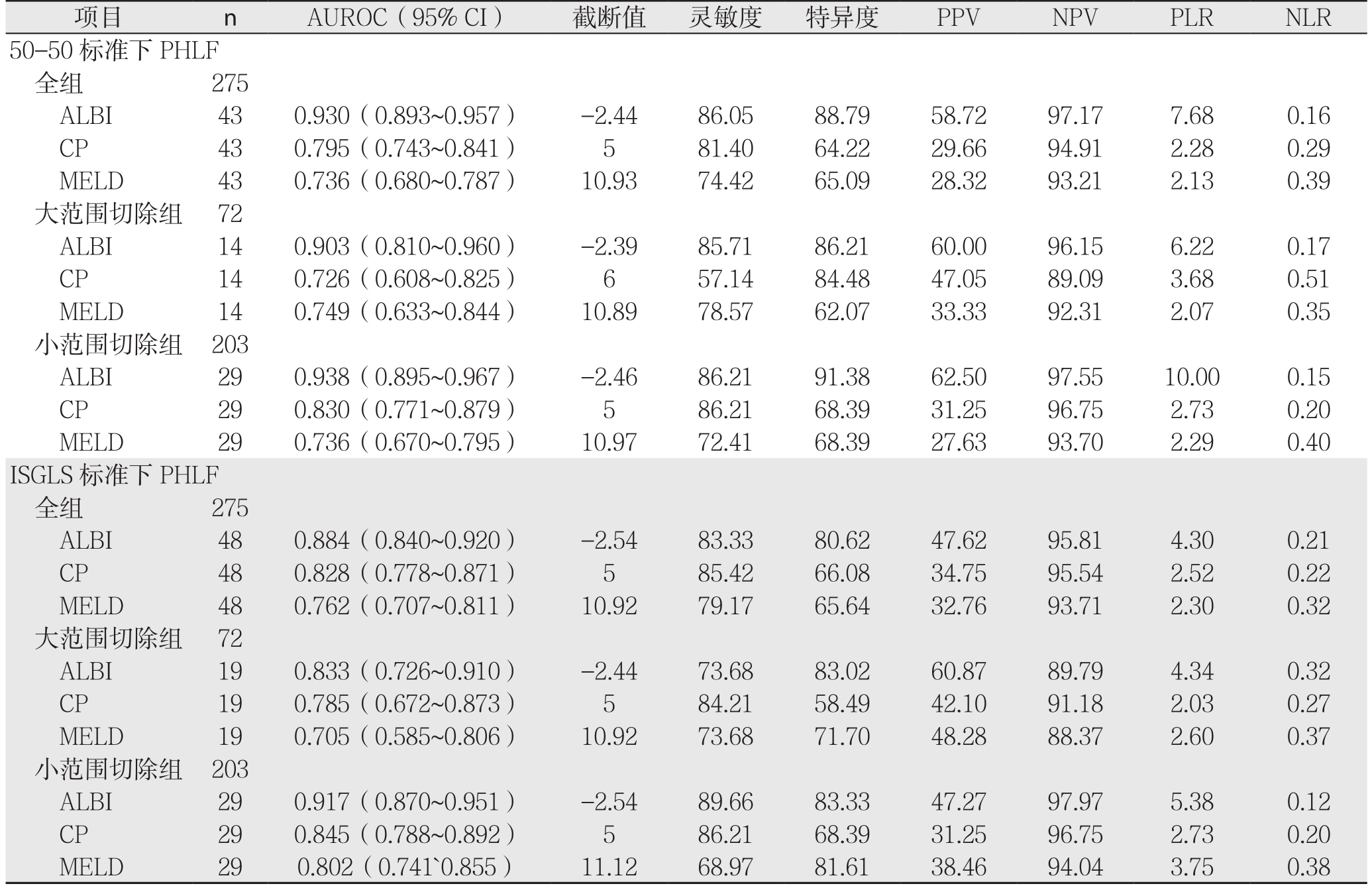
项目 n AUROC(95% CI) 截断值 灵敏度 特异度 PPV NPV PLR NLR 50-50标准下PHLF全组 275 ALBI 43 0.930(0.893~0.957) -2.44 86.05 88.79 58.72 97.17 7.68 0.16 CP 43 0.795(0.743~0.841) 5 81.40 64.22 29.66 94.91 2.28 0.29 MELD 43 0.736(0.680~0.787) 10.93 74.42 65.09 28.32 93.21 2.13 0.39大范围切除组 72 ALBI 14 0.903(0.810~0.960) -2.39 85.71 86.21 60.00 96.15 6.22 0.17 CP 14 0.726(0.608~0.825) 6 57.14 84.48 47.05 89.09 3.68 0.51 MELD 14 0.749(0.633~0.844) 10.89 78.57 62.07 33.33 92.31 2.07 0.35小范围切除组 203 ALBI 29 0.938(0.895~0.967) -2.46 86.21 91.38 62.50 97.55 10.00 0.15 CP 29 0.830(0.771~0.879) 5 86.21 68.39 31.25 96.75 2.73 0.20 MELD 29 0.736(0.670~0.795) 10.97 72.41 68.39 27.63 93.70 2.29 0.40 ISGLS标准下PHLF全组 275 ALBI 48 0.884(0.840~0.920) -2.54 83.33 80.62 47.62 95.81 4.30 0.21 CP 48 0.828(0.778~0.871) 5 85.42 66.08 34.75 95.54 2.52 0.22 MELD 48 0.762(0.707~0.811) 10.92 79.17 65.64 32.76 93.71 2.30 0.32大范围切除组 72 ALBI 19 0.833(0.726~0.910) -2.44 73.68 83.02 60.87 89.79 4.34 0.32 CP 19 0.785(0.672~0.873) 5 84.21 58.49 42.10 91.18 2.03 0.27 MELD 19 0.705(0.585~0.806) 10.92 73.68 71.70 48.28 88.37 2.60 0.37小范围切除组 203 ALBI 29 0.917(0.870~0.951) -2.54 89.66 83.33 47.27 97.97 5.38 0.12 CP 29 0.845(0.788~0.892) 5 86.21 68.39 31.25 96.75 2.73 0.20 MELD 29 0.802(0.741`0.855) 11.12 68.97 81.61 38.46 94.04 3.75 0.38
2.2.1 全组患者 3种评分分别预测PHLF所得AUROC(95% CI)及诊断结果见表3。3种评分在50-50标准下预测全组患者PHLF的ROC曲线及其相互比较,其中ALBI与CP之间面积差异为0.1350,标准误0.0374,预测能力存在统计学差异(Z=3.611,P=0.0003);ALBI与MELD之间面积差异为0.1940,标准误0.0470,预测能力存在统计学差异(Z=4.127,P=0.0001);CP与MELD之间面积差异为0.0589,标准误0.0504,预测能力无统计学差异(Z=1.170,P=0.2420)(图1)。
2.2.2 亚组分析 ⑴ 大范围切除组:3种评分分别预测PHLF所得AUROC(95%CI)及诊断结果见表3。3种评分在50-50标准下预测大范围切除组PHLF的ROC曲线及其相互比较,其中ALBI与CP之间面积差异为0.177,标准误0.737,预测能力存在明显差异(Z=2.399,P=0.0164);ALBI与MELD之间面积差异为0.154,标准误0.0762,预测能力存在统计学差异(Z=2.021,P=0.0433);CP与MELD之间面积差异为0.0228,标准误0.114,预测能力无统计学差异(Z=0.200,P=0.8418)(图2)。⑵ 小范围切除组:3种评分分别预测PHLF所得AUROC(95% CI)及诊断结果见表3。3种评分在50-50标准下预测小范围切除组PHLF的ROC曲线及其相互比较,其中ALBI与CP之间积差异为0.108,标准误0.0435,预测能力存在统计学差异(Z=2.489,P=0.0128);ALBI与MELD之间积差异为0.202,标准误0.0595,预测能力存在统计学差异(Z=3.395,P=0.0007);CP与MELD之间面积差异为0.0937,标准误0.0515,预测能力无统计学差异(Z=1.819,P=0.0690)(图3)。
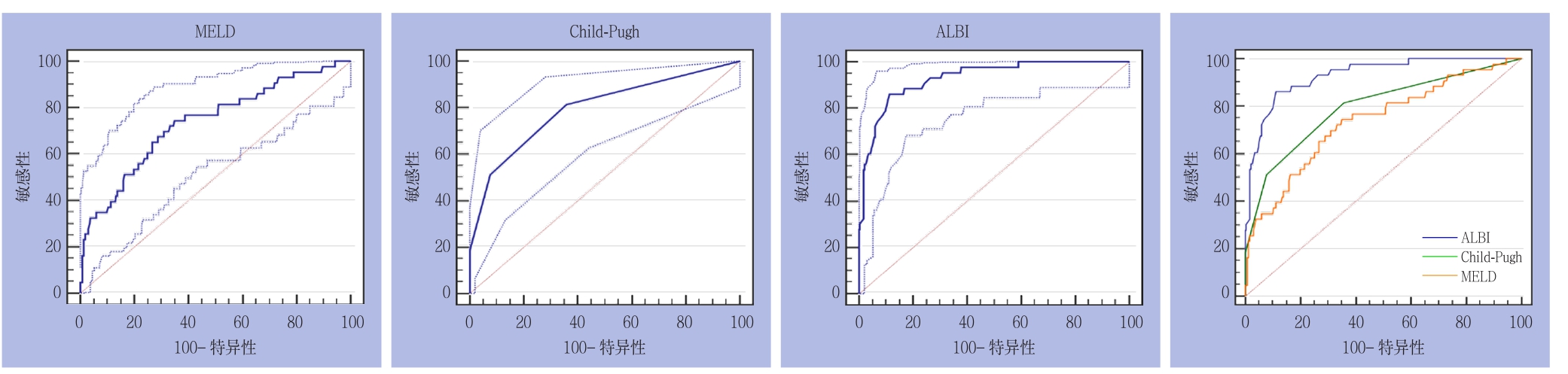
图1 3种评分在50-50标准下预测全组患者PHLF的ROC曲线
Figure 1 ROC curves of three scoring systems for predicting PHLF in the entire group according to the 50-50 criteria
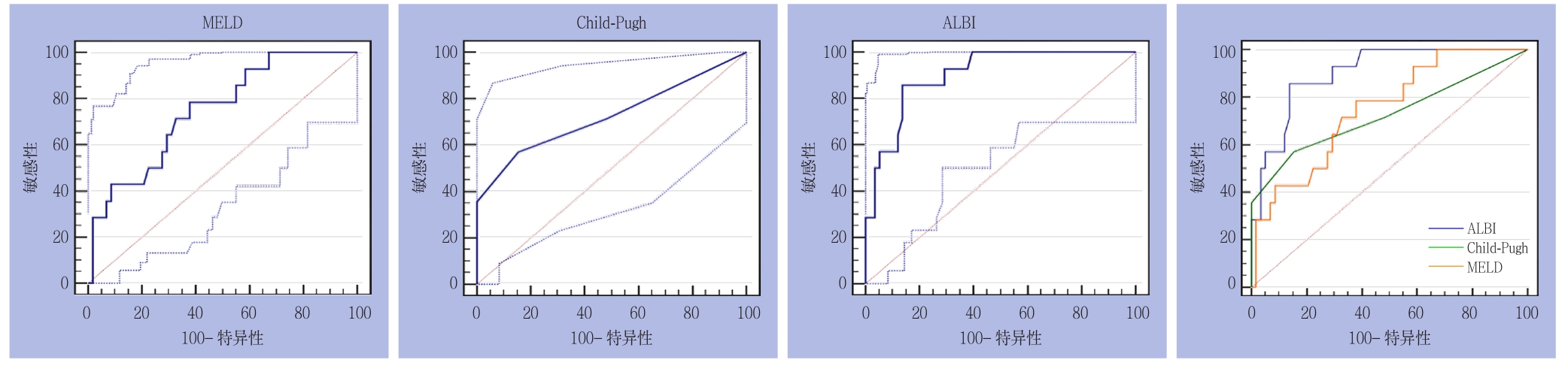
图2 3种评分在50-50标准下预测大范围切除组PHLF的ROC曲线
Figure 2 ROC curves of three scoring systems for predicting PHLF in major liver resection group according to the 50-50 criteria
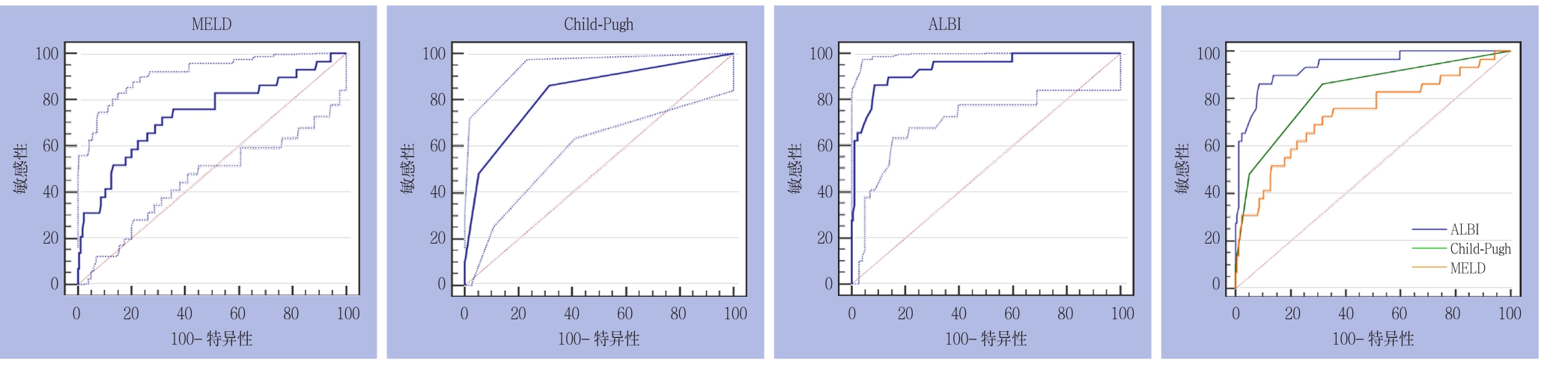
图3 3种评分在50-50标准下预测小范围切除组PHLF的ROC曲线下面积
Figure 3 ROC curves of three scoring systems for predicting PHLF in minor liver resection group according to the 50-50 criteria
2.3 ISGLS标准下3种评分预测PHLF
ISGLS标准下PHLF诊断结果显示,共48例被诊断为术后出现肝功能衰竭,其中大范围切除组中检出19例,小范围切除组中检出29例(表3)。
2.3.1 全组患者 3种评分分别预测PHLF所得AUROC(95% CI)及诊断结果见表3。3种评分在ISGLS标准下预测全组患者PHLF的ROC曲线及其相互比较,其中ALBI与CP之间面积差异为0.0561,标准误0.0394,预测能力无统计学差异(Z=1.425,P=0.1542);ALBI与MELD之间面积差异为0.1220,标准误0.0448,预测能力存在统计学差异(Z=2.727,P=0.0064);CP与MELD之间面积差异为0.0660,标准误0.0516,预测能力无统计学差异(Z=1.279,P=0.2010)(图4)。
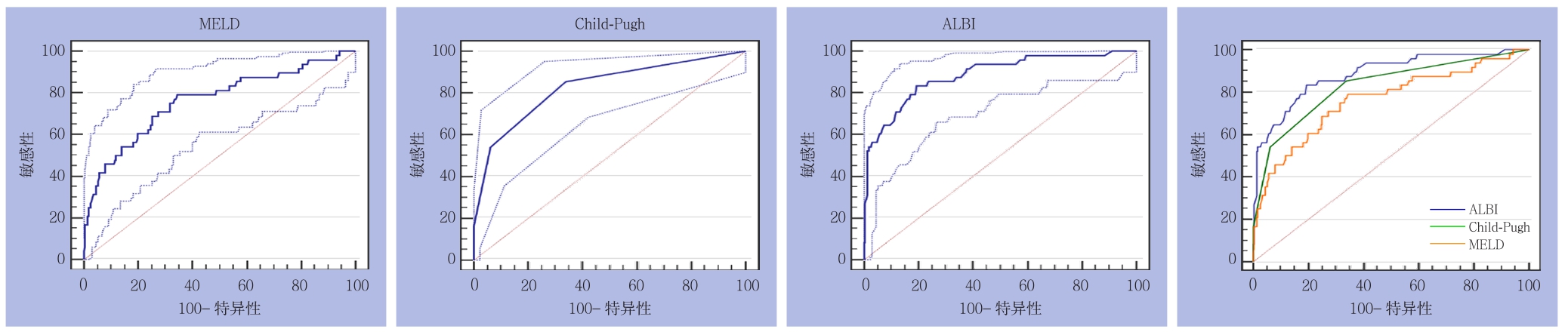
图4 3种评分在ISGLS标准下预测全组患者PHLF的ROC曲线
Figure 4 ROC curves of three scoring systems for predicting PHLF in the entire group according to the ISGLS criteria
2.3.2 亚组分析 ⑴ 大范围切除组:3种评分分别预测PHLF所得AUROC(95% CI)及诊断结果见表3。3种评分在ISGLS标准下预测大范围切除组PHLF的ROC曲线及其相互比较,其中ALBI与CP之间面积差异为0.0482,标准误0.0730,预测能力无明显差异(Z=0.659,P=0.5096);ALBI与MELD之间面积差异为0.128,标准误0.0788,预测能力无明显差异(Z=1.625,P=0.1042);CP与MELD之间面积差异为0.0799,标准误0.102,预测能力无明显差异(Z=0.781,P=0.4347)(图5)。⑵ 小范围切除组:3种评分分别预测PHLF所得AUROC(95% CI)及诊断结果见表3。3种评分在ISGLS标准下预测小范围切除组PHLF的ROC曲线及其相互比较,其中ALBI与CP之间面积差异为0.0722,标准误0.0455,预测能力无明显差异(Z=1.588,P=0.1123);ALBI与MELD之间积差异为0.1150,标准误0.0537,预测能力存在明显差异(Z=2.140,P=0.0323);CP与MELD之间面积差异为0.0427,标准误0.0542,预测能力无明显差异(Z=0.789,P=0.4303)(图6)。
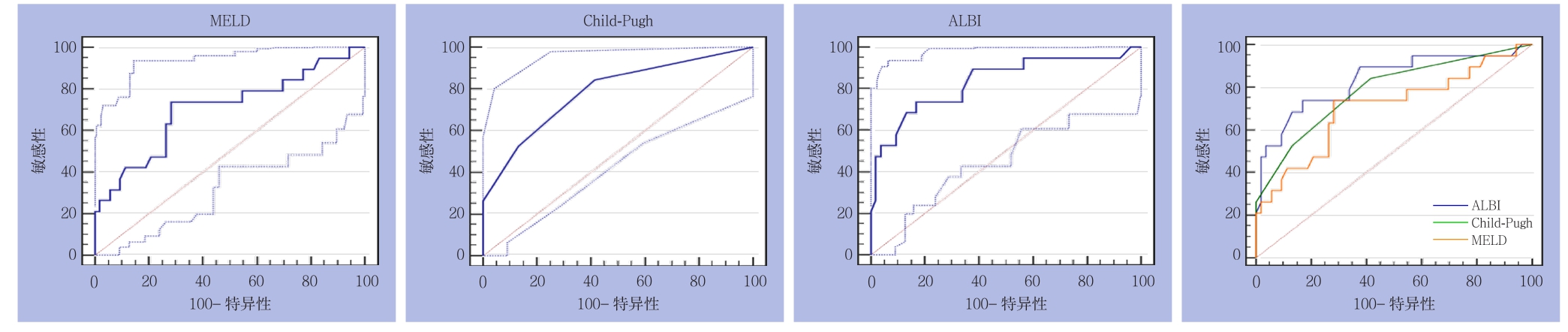
图5 3种评分在ISGLS标准下预测大范围切除组PHLF的ROC曲线
Figure 5 ROC curves of three scoring systems for predicting PHLF in major liver resection group according to the ISGLS criteria
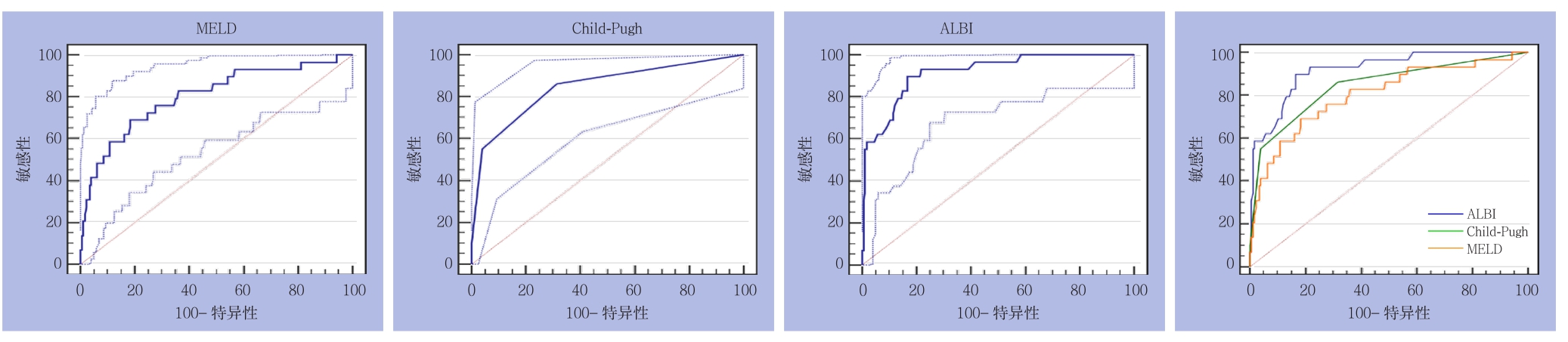
图6 3种评分在ISGLS标准下预测小范围切除组PHLF的ROC曲线
Figure 6 ROC curves of three scoring systems for predicting PHLF in minor liver resection group according to the ISGLS criteria
3 讨 论
肝癌是严重威胁人类健康的恶性肿瘤之一,近年来发病率逐年上升,我国肝癌年发病率居肿瘤发病率第3位,病死率居第2位[17-18]。目前肝癌治疗方法众多,但以根治为目的的外科手术切除仍为治疗首选,大量研究[19-21]显示肝癌R0切除术能有效提高肝癌患者的生存率。而PHLF是一种严重的肝切除术后并发症且可导致较高的病死率[22-23]。多数肝癌患者存在相关的慢性肝脏疾病,如肝硬化、腹水、门脉高压等,是PHLF的主要危险因素。因此,术前准确评估肝脏功能是降低PHLF发病率和病死率的重要手段。CP评分以及MELD评分一直被广泛应用于临床以评估肝病患者的预后,而ALBI评分自报道以来,不少研究显示该评分在某些肝脏疾病中的表现更优于前两者[24-26],因此探讨3种评价体系在PHLF中的应用有一定的临床价值。
依据50-50和ISGLS两种标准对PHLF进行诊断,以避免单一标准造成的诊断误差。每种诊断结果依据肝脏切除体积细分为大范围肝切除组和小范围切除组进行亚组分析,分别计算ALBI,CP和MELD 3种评分的ROC曲线下面积,结果显示,两种标准下的全体样本中,ALBI评分的AUROC均最大,分别为0.930(0.893~0.957)和0.884(0.840~0.920),一般认为AUROC 0.5~0.7诊断价值较低,0.7~0.9诊断价值中等,>0.9诊断价值较高,可见ALBI评分对于PHLF的诊断较准确,且在大范围肝切除组及小范围切除组中均可得到相同的结论,更说明ALBI评分具有不错的诊断效能,这可能与ALBI评价体系的产生主要基于统计学证据(大样本Cox回归模型)而非临床观察有关[5]。
CP评分在临床上作为一种评价肝功能的手段已被使用了超过40年,本研究中,两种标准下CP评分的AUROC分别为0.795(0.743~0.841)和0.828(0.778~0.871),诊断价值中等,精确度低于ALBI,略高于MELD评分。其截断值在5、6间波动,而CP评分的最低分仅为5,这表明CP评分对于轻症患者的区分度较低。且大多早期肝癌患者仅表现出乏力,食欲减退等临床症状,中晚期才表现为腹水及肝性脑病,本研究中出现腹水症状的患者仅22.5%,无1例出现肝性脑病,这无疑会降低CP评分对于早期肝癌患者预后的预测能力。
MELD评分在本研究中两种标准下的AUROC分别为0.736(0.680~0.787)和0.762(0.707~0.811),诊断价值偏低。该评分过去主要被用于肝脏移植和供体分配[27],大多在中晚期肝病中被运用,其分数构成中肌酐占有不小的权重,而肌酐是主要反映肾功能的指标,早期肝癌且无肾功能障碍的患者似乎并不适用于该评价体系。
在3种评分的相互比较中,50-50标准下,不论是大部分切除组还是局部切除组,ALBI评分的曲线下面积均显著大于另两者,而CP评分曲线下面积虽略高于MELD评分,但差异无显著意义;ISGLS标准下,虽仅全体样本及局部切除组中ALBI评分对比MELD表现出显著差异,但整体趋势与50-50标准中得到的结论一致。
综上所述,3种预测模型中,ALBI评分预测肝癌患者PHLF能力最强,CP评分其次,MELD评分预测精度较低,相对不适用于轻症早期肝癌患者。但由于本研究收纳患者均为早中期肝癌患者,且绝大部分为乙肝相关性肝癌,不能排除病因异质性对实验结果的影响,故仍需多病因,囊括中晚期肝癌患者的大样本研究来证实本研究的结论。
参考文献
[1] Gruttadauria S, Vasta F, Minervini MI, et al. Significance of the eあective remnant liver volume in major hepatectomies[J]. Am Surg,2005, 71(3):235–240.
[2] Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts[J]. Hepatology, 2000, 31(4):864–871.
[3] Forman LM, Lucey MR. Predicting the prognosis of chronic liver disease: an evolution from child to MELD. Mayo End‐stage Liver Disease[J]. Hepatology, 2001, 33(2):473–475.
[4] 张洁, 卢放根, 欧阳春晖, 等. Child‐Pugh分级和MELD评分对死亡的肝硬化患者的回顾性分析[J]. 中南大学学报:医学版, 2012,37(10):1021–1025. doi:10.3969/j.issn.1672–7347.2012.10.009.Zhang J, Lu FG, Ouyang CH, et al. Respective analysis of dead patients with cirrhosis by Child‐Pugh score and model of end-stage liver disease score[J]. Journal of Central South University:Medical Science, 2012, 37(10):1021–1025. doi:10.3969/j.issn.1672–7347.2012.10.009.
[5] Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence‐based approach‐the ALBI grade[J]. J Clin Oncol, 2015, 33(6):550–558. doi: 10.1200/JCO.2014.57.9151.
[6] Hermanek P, Wittekind C. The pathologist and the residual tumor (R)classification[J]. Pathol Res Pract, 1994, 190(2):115–123.
[7] Berzigotti A, Reig M, Abraldes JG, et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta‐analysis[J]. Hepatology,2015, 61(2): 526–536. doi: 10.1002/hep.27431.
[8] Pol B, Campan P, Hardwigsen J, et al. Morbidity of major hepatic resections: a 100‐case prospective study[J]. Eur J Surg, 1999,165(5):446–453.
[9] 中华医学会外科学分会肝脏外科学组. 肝脏解剖和肝切除手术命名及肝切除术中控制出血方法和选择原则(2017年第1次修订,第2版)[J]. 腹部外科, 2017, 30(2):75–78.Hepatic Surgery Group of Society of Surgery of Chinese Medical Association. Nomenclature of liver anatomy and resection and bleeding control methods and selection principle (First Revison 2017, Second Edition)[J]. Journal of Abdominal Surgery, 2017,30(2):75–78.
[10] Rahbari NN, Garden OJ, Padbury R, et al. Post‐hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS)[J]. HPB (Oxford), 2011,13(8):528–535. doi: 10.1111/j.1477–2574.2011.00319.x.
[11] Balzan S, Belghiti J, Farges O, et al. The "50–50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy[J]. Ann Surg, 2005, 242(6):824–828.
[12] 王宪强. 肝细胞癌肝切除的决策树安全性分析和影响因素研究[D]. 济南: 山东大学, 2015.Wang XQ. Safe valadition of diあerent decision trees and risk factors for hepatectomy in hepatocellular patients[D]. Jinan: Shandong University, 2015.
[13] Shen Y, Zhou C, Zhu G, et al. Liver Stiffness Assessed by Shear Wave Elastography Predicts Postoperative Liver Failure in Patients with Hepatocellular Carcinoma[J]. J Gastrointest Surg, 2017, doi:10.1007/s11605–017–3443–9. [Epub ahead of print]
[14] 文静, 张维, 赫嵘, 等. 50–50标准联合肝功能衰竭分级在肝癌肝切除术后护理中的应用研究[J]. 护士进修杂志, 2017, 32(4):300–303. doi:10.16821/j.cnki.hsjx.2017.04.004.Wen J, Zhang W, He R, et al. Clinical research on post hepatectomy nursing care with "50–50 criteria" and PHLF grading for hepatocellular carcinoma patients[J]. Journal of Nurses Training,2017, 32(4):300–303. doi:10.16821/j.cnki.hsjx.2017.04.004.
[15] Pugh RN, Murray‐Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices[J]. Br J Surg, 1973,60(8):646–649.
[16] Kamath PS, Kim WR. The model for end‐stage liver disease(MELD)[J]. Hepatology, 2007, 45(3):797–805.
[17] 陈万青, 郑荣寿, 张思维, 等. 2012年中国恶性肿瘤发病和死亡分析[J]. 中国肿瘤, 2016, 25(1):1–8. doi:10.11735/j.issn.1004–0242.2016.01.A001.Chen WQ, Zheng RS, Zhang SW, et al. Report of Cancer Incidence and Mortality in China, 2012[J]. China Cancer, 2016, 25(1):1–8.doi:10.11735/j.issn.1004–0242.2016.01.A001.
[18] Balogh J, Victor D 3rd, Asham EH, et al. Hepatocellular carcinoma:a review[J]. J Hepatocell Carcinoma, 2016, 3:41–53.
[19] Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced‐stage hepatocellular carcinoma[J]. Ann Surg, 2014, 260(2):329–340.doi: 10.1097/SLA.0000000000000236.
[20] Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study[J]. J Hepatol, 2015, 62(3):617–624. doi: 10.1016/j.jhep.2014.10.037.
[21] Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade[J]. Ann Surg, 2002, 236(4):397–406.
[22] van den Broek M A, Olde Damink S W, Dejong C H, et al. Liver failure after partial hepatic resection: definition, pathophysiology,risk factors and treatment[J]. Liver Int, 2008, 28(6):767–80. doi:10.1111/j.1478–3231.2008.01777.x.
[23] 陈平, 何振平, 韩本立, 等. 21例原发性肝癌患者术后死亡原因分析[J]. 普外基础与临床杂志, 1997, 4(1):30–32.Chen P, He ZP, Han BL, et al. The causes of death for the primary licer cancer after operation of 21 cases[J]. Chinese Journal of Bases and Clinics in General Surgery, 1997, 4(1):30–32.
[24] Chan AW, Chan RC, Wong GL, et al. New simple prognostic score for primary biliary cirrhosis: Albumin‐bilirubin score[J].J Gastroenterol Hepatol, 2015, 30(9):1391–1396. doi: 10.1111/jgh.12938.
[25] Li MX, Zhao H, Bi XY, et al. Prognostic value of the albumin‐bilirubin grade in patients with hepatocellular carcinoma: Validation in a Chinese cohort[J]. Hepatol Res, 2017, 47(8):731–741. doi:10.1111/hepr.12796.
[26] Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era[J]. J Gastroenterol Hepatol, 2017, 32(4):879–886.doi: 10.1111/jgh.13608.
[27] Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child‐Pugh versus MELD[J]. J Hepatol, 2005, 42(Suppl 1):S100–107.
