自1962年Jensen等[1]成功分离出雌激素受体(estrogen receptor,ER),奠定了乳腺癌内分泌治疗的分子基础,长期以来的临床实践确立了乳腺癌内分泌治疗的金标准——三苯氧胺(tamoxifen,TAM)的一线治疗地位,并使众多患者获益。然而有学者[2-3]发现40%初始抗雌激素治疗有效的ER阳性乳腺癌患者,随着疗程的延长会出现继发耐药,而且一旦发生耐药,雌激素受体调节类药物(SERMs)反而会促进肿瘤的生长。获得性雌激素受体抑制剂耐药的问题日益严重,第32届圣安东尼奥乳腺癌大会(SABCS,2009年)首次报道醛糖还原酶抑制剂可以逆转内分泌耐药细胞的耐药性,初步阐明其机制是通过上调ERα及下调HER-2/MAPK的水平[4]。槲皮素(quercetin,QUE)是一种天然植物来源的黄酮类醛糖还原酶抑制剂,具有多种抗肿瘤活性,其机制可能是其具有抑制多种肿瘤细胞的MAPK、Akt信号系统活性[5-7],对乳腺癌细胞,QUE能够下调HER-2的表达[8]。本实验以耐TAM人乳腺癌荷瘤裸鼠为主要研究对象,从实验动物水平观察QUE诱导下TAM对移植瘤的疗效,并初步探讨其作用的分子机制,以期为临床治疗提供一个新的思路和方法。
1 材料与方法
1.1 实验材料
人乳腺癌细胞MCF-7购自中科院上海细胞资源中心,SPF级BALB/c-nu雌性裸小鼠购自中山大学实验动物中心,胎牛血清、DMEM培养液和0.25%胰蛋白酶购于Gibico公司,QUE、4-羟三苯氧胺(4-hydroxytamoxifen,4-OH-TAM)购自Sigma公司,枸橼酸三苯氧胺(tamoxifen citrate tablets)为阿斯利康公司产品,RIPA蛋白裂解液(强)、PMSF蛋白酶抑制剂、Bradford蛋白浓度测定试剂盒等为碧云天公司产品。抗-ERα、抗-actin购自Santa Cruz,抗-pAkt、抗-MAPK购自Cell Signaling公司,抗-HER-2、抗-Akt、抗-pMAPK、羊抗兔IgG-HRP等购自CST。
1.2 构建TAM耐药乳腺癌细胞株
常规培养雌激素受体阳性的人乳腺癌细胞株MCF-7,采用高浓度短时间,4-OH-TAM冲击法[9]诱导人乳腺癌TAM耐药细胞株MCF-7/TAM-R:取对数生长期细胞接种于直径10 cm培养皿中,观察细胞生长状态良好,加入4-OH-TAM(终浓度10-6mol/L)并每2~3天换液(含等浓度4-OH-TAM)1次。在上述培养环境培养21 d,收获乳腺癌TAM耐药细胞株MCF-7/TAM-R,并在无药物干预下扩增,在含耐药维持浓度为10-7mol/L的4-OH-TAM培养液中维持MCF-7/TAM-R的耐药性。
1.3 TAM耐药乳腺癌动物模型的构建及分组
1.3.1 建立人TAM耐药乳腺癌动物模型 ⑴ 细胞接种法制备裸鼠皮下移植瘤模型:BALB/c-nu雌性裸小鼠 3只,4~5周龄,体质量 14~18 g,在SPF屏障系统(恒温、恒湿、无菌、净化)中饲养;取对数生长期的人乳腺癌TAM耐药细胞MCF-7/TAM-R,常规胰酶消化后吹打成单细胞悬液,800 r/min离心5 min收集细胞,PBS液清洗2次并重悬细胞,调整细胞浓度至5.0×107/mL,每只裸鼠接种0.2 mL MCF-7/TAM-R单细胞悬液(含细胞5×106个)于一侧背部皮下形成皮丘(避免注射到皮内,穿刺点无液体溢出),观察接种部位肿瘤生长状况,以瘤块直径达1.0 cm为造模标准。⑵ 组织块法制备裸鼠皮下移植瘤模型:细胞接种法制备皮下移植瘤模型成功,断颈处死裸鼠,无菌环境中完整剥离皮下肿瘤作为移植瘤源,剔除周围结缔组织及肿瘤部分坏死组织。取生长活跃的肿瘤边缘组织,无菌生理盐水漂洗后,眼科剪修剪为约2~3 mm3的小组织块,置无菌生理盐水中备用。取4~5周龄BALB/c-nu雌性裸小鼠24只,体质量14~18 g,消毒右侧颈背部皮肤,眼科剪剪开一长约2~4 mm小口,适当游离皮下疏松组织形成一小隧道;眼科镊夹取备好的肿瘤小组织块置入皮下,轻轻压迫、闭合皮肤切口(图1)。观察肿瘤生长情况及致瘤率,以瘤体直径超过0.5 cm为成瘤标准。

图1 组织块法制备裸鼠皮下移植瘤
Figure 1 Subcutaneous tumor transplantation with tumor tissue blocks
1.3.2 荷瘤动物分组 达成瘤标准后,将荷瘤裸鼠随机分为4组,每组6只。⑴ 对照组:二甲基亚砜(DMSO,QUE的空白溶媒,等容积腹腔内注射,1次/2 d)+生理盐水(TAM的空白溶媒,等容积灌服,1次/1 d) 处 理, 至 第21天。 ⑵ QUE组:QUE(50 mg/kg,DMSO稀释腹腔内注射,1次/2 d)[10]+生理盐水(TAM的空白溶媒,等容积灌服,1次/1 d)处理,至第21天;⑶ TAM组:TAM(5 mg/k,生理盐水稀释灌服,1次/1 d)[11]+DMSO(腹腔内注射,1次/2 d)处理,至第21天;⑷ QUE+TAM组:QUE(50 mg/kg,DMSO稀释腹腔内注射,1次/2 d)+TAM(5 mg/kg,生理盐水稀释灌服,1次/1 d)处理,至第21天。
1.3.3 各组荷瘤裸鼠体质量、瘤体体积与瘤体质量 注药前、注药后每3天测量各组荷瘤裸鼠体质量,绘制体质量曲线;用游标卡尺测量肿瘤的最长径(a)和与之垂直的最短径(b),根据公式计算瘤体体积:V(cm3)=ab2×0.52[12],绘制瘤体生长曲线;21 d后拉颈处死裸鼠,剥离皮下肿瘤,测量瘤体质量。
1.4 Western blot检测瘤组织ERα、HER-2、pMAPK、MAPK以及pAkt、Akt蛋白表达
常规RIPA及PMSF提取各组瘤体组织总蛋白,12%SDS-PAGE电泳分离后,转至硝酸纤维素膜上,置5%的脱脂奶粉-PBST封闭液中室温封闭2 h,分别于稀释的一抗:抗-ERα、抗-HER-2、抗-pMAPK、抗-MAPK以及抗-pAkt、抗-Akt中4 ℃过夜。用辣根过氧化物酶偶联的二抗(羊抗兔IgG-HRP)平稳摇动、室温孵育2 h,ECL显色,置于X光片盒中,压片曝光。
1.5 统计学处理
采用SPSS 19.0统计软件,统计数据用均数±标准差(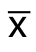 ±s)表示,单因素方差分析进行统计学处理,两组间比较用LSD法,P<0.05为差异有统计学意义。
±s)表示,单因素方差分析进行统计学处理,两组间比较用LSD法,P<0.05为差异有统计学意义。
2 结 果
2.1 TAM耐药乳腺癌细胞株细胞制备及模型成功率
用4-OH-TAM高浓度(10-6mol/L)、短时间持续冲击方法成功构建MCF-7/TAM-R细胞。在4-OH-TAM筛选初期,即筛选的第5~10天,MCF-7细胞的增殖活力明显受抑,可以观察到有较多的MCF-7细胞死亡;而到了筛选后期几乎无新的细胞死亡发生。筛选获得的4-OH-TAM耐药细胞株与亲代MCF-7细胞从形态上比较,没有明显差别(图2),MCF-7/TAM-R构建成功。
将瘤源组织块置入裸鼠右侧颈背部皮肤皮下,1周内全部达成瘤标准,成瘤率为100%(24/24)。
图2 亲代MCF-7细胞和TAM耐药MCF-7细胞形态观察(×100)
Figure 2 Morphologic observation of the parent MCF-7 and TAM-resistant MCF-7 (×100)
2.2 各组荷瘤裸鼠的体质量、瘤体体积与瘤体质量变化情况
2.2.1 体质量变化 在用药前期,即用药的前12 d,各组动物摄食良好、活动正常,体质量逐渐增长,TAM组动物体质量增长相对缓慢,但无统计学差异(P>0.05)。2周后QUE+TAM组和QUE组裸鼠摄食减少、体质量减轻,动物消瘦、行动迟缓;第18~21天QUE+TAM组动物体质量明显减轻(均P<0.05),出现反应迟钝、萎靡,部分动物濒临死亡;相对于QUE+TAM组,上述表现在QUE组动物稍轻。而对照组、TAM组动物饮食、活动正常,体质量无明显变化(P>0.05)(图3)。
2.2.2 瘤体体积 对照组、TAM组、QUE组动物的瘤体呈持续增长,其中以TAM组瘤体增长最为迅速,但无统计学差异(P>0.05);而QUE组增长速度相对缓慢、瘤体较小,但亦无统计学差异(P>0.05)。QUE+TAM组在用药的前12 d,瘤体呈逐渐增长趋势,与各对照组基本一致;12~15 d增长速度接近于平台期;而到实验后期(18~21 d),瘤体生长呈下降趋势,逐渐变小,差异有统计学意义(P<0.05)(图4)。
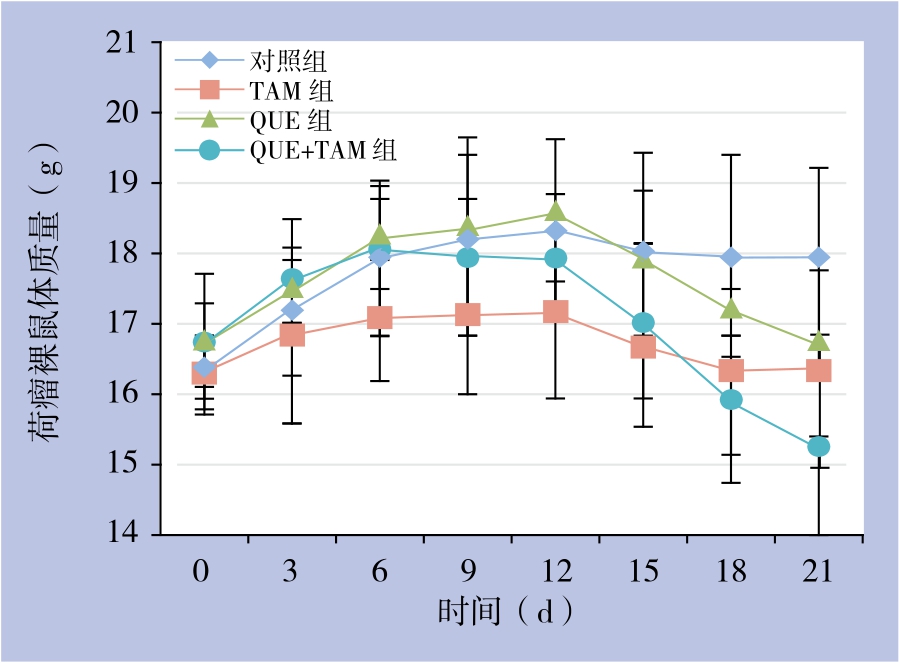
图3 各组荷瘤裸鼠体质量变化
Figure 3 Changes of body weight of the tumor-bearing nude mice in each group
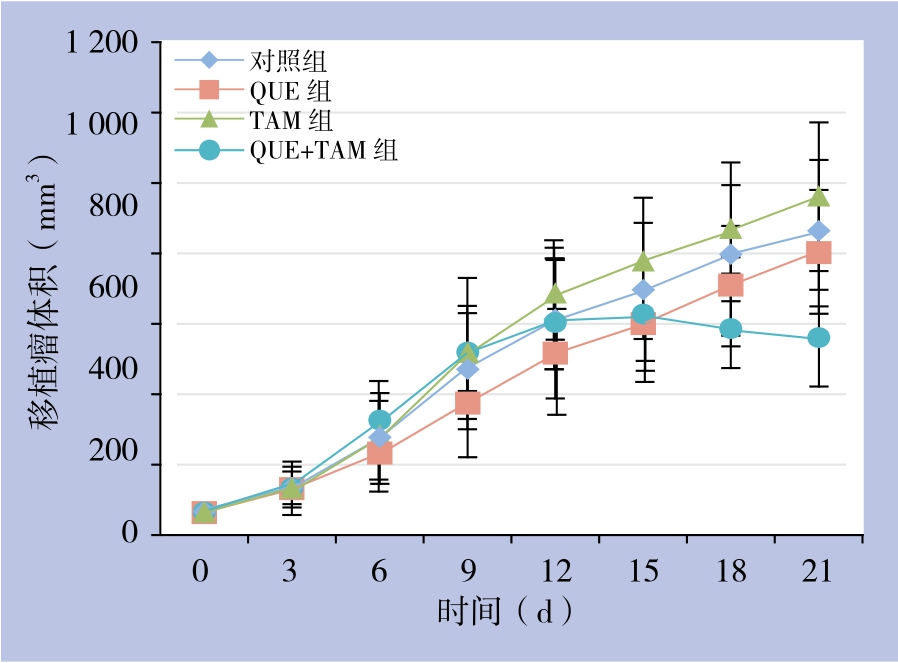
图4 各组荷瘤鼠移植瘤生长趋势
Figure 4 Growth tendency of the xenografts in each groups of mice
2.2.3 瘤体质量 在给药后第21天,24只动物均存活,大体解剖未发现远处转移灶,完整剥离皮下肿瘤、称重(图5A)。与其余各组比较,QUE+TAM组的瘤体质量明显降低(均P<0.05);其余各组间比较差异无统计学意义(P>0.05),尽管没有统计学差异,但QUE组的瘤体质量小于其他两组(图5B)。
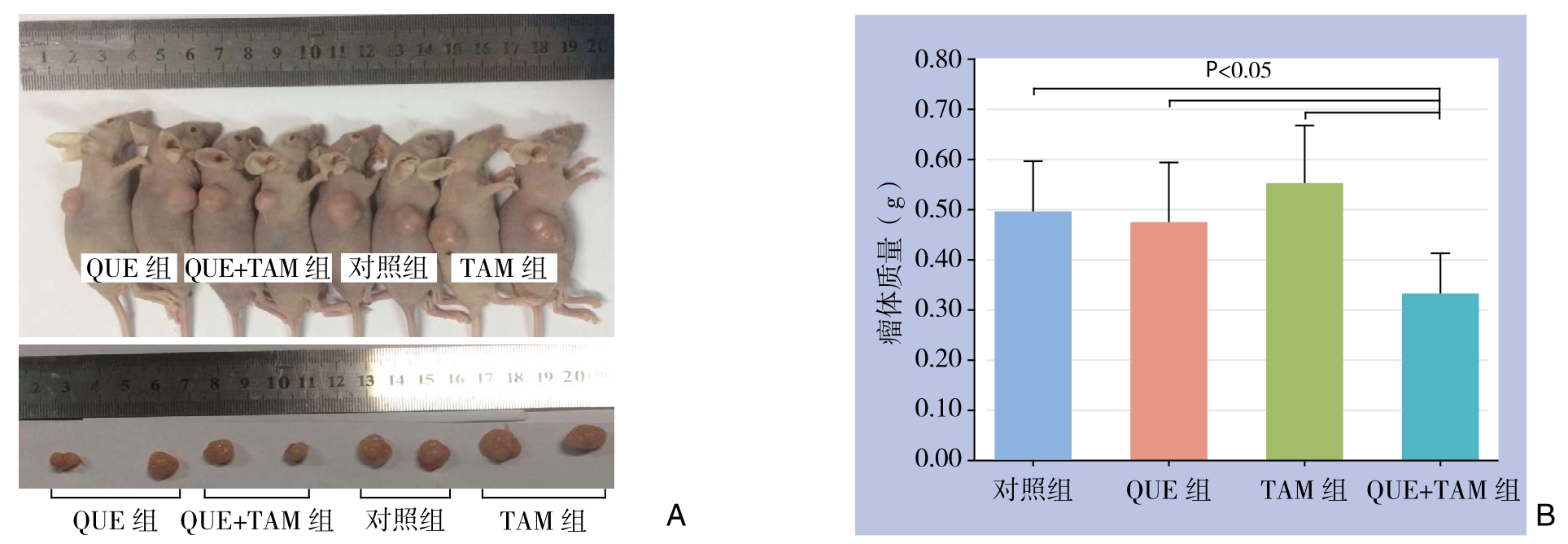
图5 给药后第21天各组移植瘤情况
Figure 5 Xenografts in each group at the 21th day of treatment
A:大体标本;B:各组瘤体质量比较
A: Gross specimens; B: Comparison of the tumor weights among groups
2.3 瘤组织中ERα、HER-2、pMAPK、MAPK、pAkt、Akt蛋白表达水平
Western blot结果显示,与对照组比较,QUE+TAM组和QUE组瘤组织中ERα蛋白的表达明显升高,而HER-2、pERK、pAkt蛋白的表达明显降低,而TAM组以上蛋白表达无明显差异;非酸化的ERK、Akt蛋白表达水平在各组间均无明显差异(图6)。

图6 瘤组织中ERα、HER-2、pMAPK、pAkt、MAPK、Akt蛋白表达检测
Figure 6 Determination of protein expressions of ERα, HER-2,pMAPK, pAkt, MAPK and Akt
3 讨 论
乳腺癌内分泌治疗耐药的主要原因是ERα和HER-2信号通路的串话调节(cross talk)。抑制一个信号通路,可使肿瘤细胞在强大的生存压力下激活另一个信号通路,得以继续增殖、侵袭和迁移,产生耐药性。生长因子信号通路的异常激活,使其下游信号通路Ras-Raf-MEK-ERK通路和PI3K-Akt-mTOR通路的过度活化,活化的ERK和Akt激酶促使ER的AF-1及其共调节因子的关键位点磷酸化,出现不依赖配体的ER激活途径,是内分泌治疗耐药的非配体依赖性活性的主要通路,导致肿瘤细胞逃逸[13-15]。
探讨HER-2或其下游信号通路Ras-Raf-MEKERK和PI3K-Akt-mTOR的信号通路的抑制剂,恢复内分泌治疗的敏感性迫在眉睫。Miller等[16]的一项体外实验显示,用HER-2的单克隆抗体曲妥珠单抗或拉帕替尼抑制乳腺癌细胞中HER-2的表达,能够增强ER转录活性,促使ER表达上调,恢复细胞对内分泌药物的敏感性。在来曲唑长时间处理的乳腺癌细胞LTLT-Ca中,Jelovac等[17]发现该细胞系中HER-2、p-Raf、p-MEK1/2及p-MAPK表达上调,以致细胞耐药后磷酸化ER水平提高,导致非配体依赖的ER转录活性上调,虽然ER水平是下降的;而应用MAPK通路抑制剂或酪氨酸激酶抑制剂吉非替尼,LTLT-Ca细胞的生长受到抑制,对来曲唑的敏感性恢复。Macedo等[18]发现应用曲妥株单抗能恢复ER的表达水平,并恢复LTLT-Ca细胞对内分泌治疗的敏感性。PI3K-Akt-mTOR信号通路研究最为成熟的是哺乳动物雷帕霉素靶蛋白(mTOR)抑制剂依维莫司。Bachelot等[19]的研究发现,依维莫司联合TAM能显著改善乳腺癌内分泌治疗的继发耐药。而在芳香化酶抑制剂治疗失败的ER阳性转移性乳腺癌中,依维莫司联合TAM能显著延长无进展生存时间(PFS)和总生存期(OS)[20]。
从天然植物来源中寻找有效逆转TAM耐药的化合物,是一种较为理想的选择。QUE一种天然植物来源的黄酮类活性小分子,能够抑制多种肿瘤细胞MAPK、Akt信号系统活性[5-7],干扰细胞信号转导、抑制肿瘤细胞增殖转移[21-23]、抗肿瘤血管生成[24]等活性。对乳腺癌细胞,QUE能够使HER-2的表达下调[8]。本研究发现,在实验的第12天,QUE+TAM组瘤体的生长呈下降趋势,体积逐渐变小,并且在结束用药后,QUE+TAM组的瘤体质量量显著降低,表明在QUE诱导下,TAM可使人乳腺癌内分泌耐药荷瘤鼠瘤体的生长显著受抑;进而,在对信号通路及分子机制的研究中发现,QUE下调耐药移植瘤组织中HER-2、pERK、pAkt的表达水平,由此阻断了HER-2与ERα的串话调节通路,并且使ERα表达上调,恢复肿瘤组织对内分泌药物的敏感性。
笔者在前期预实验中显示实验动物在每天1次腹腔内注射QUE(50 mg/kg)时,容易形成腹水,同时动物摄食、活动减少,消瘦,反应迟钝,提示QUE具有潜在的毒副作用,为保证动物存活,研究如期进行,由此改良给药频次,由文献[10]报道的“QUE(50 mg/kg)1次/1 d腹腔内注射”改良为“QUE(50 mg/kg)1次/2 d腹腔内注射”。改良后的QUE给药频次并不影响其内分泌耐药的增敏效应,但却不具有文献[10]报道的显著性抑瘤效果,只是相对地使瘤体增长速度减慢、重量减轻。从移植瘤的生长趋势上,以TAM组瘤体增长最为迅速,提示TAM可能促进对内分泌耐药乳腺癌肿瘤的生长,与Schiff等[3]的研究报道一致,但本研究中无统计学差异,需要进一步通过扩大样本量、延长给药时间或改进操作技术等研究来明确。
乳腺癌内分泌耐药带来的高复发转移率和高病死率,使得开发有效的逆转药物迫在眉睫。本文的研究提示,在QUE诱导下,TAM恢复对裸鼠乳腺癌内分泌耐药移植瘤的抑制效应,然而临床上乳腺癌TAM耐药的病因复杂,动物模型不能完全模拟出人体内复杂的内环境,临床效果及安全性方面还有待进一步论证。另外,尽管有文献[25]报道QUE对正常细胞毒性较小,仍有不少学者对QUE的药用安全性提出了质疑,本研究的结果也显示QUE能导致动物摄食减少、消瘦,失去活力,后期动物体质量明显减轻,部分动物濒临死亡,其潜在的毒副作用、安全范围及有效剂量有待进一步探讨。
参考文献
[1] Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action[J]. Recent Prog Horm Reg, 1962, 18:387–414.
[2] Tanic N, Milovanovic Z, Tanic N, et al. The impact of PTEN tumor suppressor gene on acquiring resistance to tamoxifen treatment inbreast cancer patients[J]. Cancer Biol Ther, 2012, 13(12):1165–1174. doi: 10.4161/cbt.21346.
[3] Schiff R, Massarweh S, Shou J, et al. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response[J]. Clin Cancer Res, 2003, 9(1 Pt 2):447S-454S.
[4] Tekmal R, Nair H, Huffman S, et al. Targeting Aldose Reductase:A Novel Strategy in Treating Endocrine Resistance Using Combination Therapy[J]. Cancer Res, 2009, 69(24 Suppl):Abstract nr 67. doi:10.1158/0008–5472.
[5] Ding M, Zhao J, Bowman L, et al. Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE pathway by quercitrin[J]. Int J Oncol, 2010, 36(1):59–67.
[6] Ying B, Yang T, Song X, et al. Quercetin inhibits IL-1 beta-induced ICAM-1 expression in pulmonary epithelial cell line A549 through the MAPK pathways[J]. Mol Biol Rep, 2009, 36(7):1825–1832.doi: 10.1007/s11033–008–9386–1.
[7] Nam TW, Yoo CI, Kim HT, et al. The flavonoid quercetin induces apoptosis and inhibits migration through a MAPK-dependent mechanism in osteoblasts[J]. J Bone Miner Metab, 2008, 26(6):551–560. doi: 10.1007/s00774–008–0864–2.
[8] Jeong JH, An JY, Kwon YT, et al. Quercetin-induced ubiquitination and down-regulation of HER-2/neu[J]. J Cell Biochem, 2008,105(2):585–595. doi: 10.1002/jcb.21859.
[9] Coser KR, Wittner BS, Rosenthal NF, et al. Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor[J]. Proc Natl Acad Sci U S A, 2009, 106(34):14536–14541. doi: 10.1073/pnas.0907560106.
[10] 吴凯南, 钟晓刚, 马双慰, 等. 槲皮素对人乳腺癌裸鼠移植瘤的抑制作用及其对血管生成的影响[J]. 中国肿瘤临床, 2003,30(6):434–438. doi:10.3969/j.issn.1000–8179.2003.06.016.Wu KN, Zhong XG, Ma SW, et al. Inhibitory Effect of Quercetin on Growth and Angiogenesis of Transplantation Tumor of Breast Cancer Cell line MCF-7 in Nude Mice[J]. Chinese Journal of Clinical Oncology, 2003, 30(6):434–438. doi:10.3969/j.issn.1000–8179.2003.06.016.
[11] 康欣梅, 张清媛, 王恕怀, 等. 三苯氧胺联合人参皂苷Rg3抑制乳腺癌血管生成的研究[J]. 肿瘤, 2008, 28(4):279–281. doi:10.3781/j.issn.1000–7431.2008.04.002.Kang XM, Zhang QY, Wang XH, et al. Antiangiogenic effect of tamoxifen combined with ginsenoside Rg3 on breast carcinoma[J]. Tumor, 2008, 28(4):279–281. doi:10.3781/j.issn.1000–7431.2008.04.002.
[12] Szajda SD, Jankowska A, Zwierz K. Carbohydrate markers in colon carcinoma[J]. Dis Markers, 2008, 25(4/5):233–242.
[13] Viedma-Rodríguez R, Baiza-Gutman L, Salamanca-Gómez F, et al. Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review)[J]. Oncol Rep, 2014,32(1):3–15. doi: 10.3892/or.2014.3190.
[14] Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer[J]. Annu Rev Med, 2011, 62:233–247. doi: 10.1146/annurev-med-070909–182917.
[15] Barone I, Cui Y, Herynk MH, et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway[J]. Cancer Res, 2009, 69(11):4724–4732. doi:10.1158/0008–5472.CAN–08–4194.
[16] Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer[J]. J Clin Oncol, 2011,29(33):4452–4461. doi: 10.1200/JCO.2010.34.4879.
[17] Jelovac D, Sabnis G, Long BJ, et al. Activation of mitogenactivated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole[J]. Cancer Res, 2005,65(12):5380–5389.
[18] Macedo LF, Sabnis G, Brodie A. Preclinical modeling of endocrine response and resistance: focus on aromatase inhibitors[J]. Cancer,2008, 112(3 Suppl):679–688.
[19] Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study[J]. J Clin Oncol, 2012,30(22):2718–2724. doi: 10.1200/JCO.2011.39.0708.
[20] Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer[J]. N Engl J Med, 2012, 366(6):520–529. doi: 10.1056/NEJMoa1109653.
[21] Lee YK, Park SY, Kim YM, et al. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin[J]. Exp Mol Med, 2009, 41(3):201–207. doi:10.3858/emm.2009.41.3.023.
[22] Castillo-Pichardo L, Martínez-Montemayor MM, Martínez JE, et al.Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols[J]. Clin Exp Metastasis, 2009,26(6):505–516. doi: 10.1007/s10585–009–9250–2.
[23] Touil YS, Fellous A, Scherman D, et al. Flavonoid-induced morphological modifications of endothelial cells throughmicrotubule stabilization[J]. Nutr Cancer, 2009, 61(3):310–321. doi:10.1080/01635580802521346.
[24] Ansó E, Zuazo A, Irigoyen M, et al. Flavonoids inhibit hypoxiainduced vascular endothelial growth factor expression by a HIF-1 independent mechanism[J]. Biochem Pharmacol, 2010,79(11):1600–1609. doi: 10.1016/j.bcp.2010.02.004.
[25] 黄春龙, 彭伟, 张继红, 等. quercetin抑制肝细胞癌生长的在体实验研究[J]. 中国普通外科杂志, 2015, 24(6):828–833. doi:10.3978/j.issn.1005–6947.2015.06.012.Huang CL, Peng W, Zhang JH, et al. Quercetin inhibiting growth of hepatocellular carcinoma cells: in vivo experimental study[J].Chinese Journal of General Surgery, 2015, 24(6):828–833.doi:10.3978/j.issn.1005–6947.2015.06.012.