胆管癌是一种具有很强侵袭力的恶性肿瘤,预后差,5年无瘤生存率仅为12.8%,中位生存时间仅1.5年[1],但胆管癌的发生与发展的机制尚未完全探明[2-4]。最近研究[5-6]发现,乳腺丝氨酸蛋白酶抑制物Maspin与肿瘤细胞的增殖、浸润和转移有关,因此本研究检测Maspin蛋白在原发性胆管癌中的表达及其与p53表达的相关性,并探讨其与预后的临床意义,从而为胆管癌的诊断和治疗提供新的思路。
1 材料与方法
1.1 材料
1.1.1 标本来源及处理 胆管癌组织连同癌旁组织(自距肿瘤边缘约1.0 cm的组织)42例,收集于2006年7月—2011年10月湘雅医院经过外科手术并经湘雅医院病理科明确诊断的胆管癌患者,其中男27例,女15例;年龄37~78岁,平均(61.98±9.60)岁,术前均未作放疗、化疗、免疫治疗。按WHO病理分级标准分:高分化癌23例,中分化癌11例,低分化癌8例。按病理类型分:管状腺癌25例,乳头状腺癌10例,黏液腺癌7例。按肿瘤部位分:肝门部17例,胆总管中段12例,胆总管下段13例。按淋巴结转移分:淋巴结转移17例,无淋巴结转移25例。按远处转移分:远处转移11例,无远处转移31例。正常胆管组织12例,标本均取自肝胆管结石患者行Roux-en-Y胆肠吻合的正常胆管组织,其中男7例,女5例;年龄32~75岁,平均(46.34±11.3)岁。上述组织放入10%福尔马林中固定,并包埋在石蜡块中,以备免疫组化用,蜡块作3~4 μm的切片3张,其中1张行HE常规染色,另2张分别行Maspin、p53免疫组化检测,且HE常规染色均经湘雅医院病理科高年资医生病理检查证实。
1.1.2 抗体 小鼠抗人Maspin单克隆抗体(EAW2,Cat.#MS-1767-S0), 购自美国NeoMarkers公司(Frement,CA,USA),适用的稀释浓度分别为1:10。小鼠抗人p53单克隆抗体(DO-7)购自北京中杉金桥公司,适用的稀释浓度分别为1:50。免疫组化试剂盒购自上海晶美生物技术有限公司。
1.2 方法
1.2.1 免疫组织化学步骤 采用SP法检测Maspin与p53的表达。检测方法按试剂盒说明书进行,用已知阳性的正常前列腺组织切片作为Maspin的阳性对照。以磷酸盐缓冲液代替一抗作为阴性对照。
1.2.2 结果判断 每张切片随机连续观察5个高倍视野,每处视野计数100个细胞。Maspin免疫组化染色以细胞浆或/和胞核中出现棕黄色颗粒为阳性,结果判定标准:Maspin以细胞浆和/或细胞核内出现棕黄色颗粒为阳性细胞,采用半定量积分法统计检测结果。具体评分标准[7]按⑴ 着色强度:无着色为0分,淡黄色为1分,黄色为2分,深棕黄色为3分;⑵ 阳性细胞数:无阳性细胞为0分,<25%为1分,25%~50%为2分,>50%为3分。⑴⑵项相加,0~2分为阴性(-),3~4分为阳性(+),5~6分为强阳性(++)。p53免疫组化染色以细胞核中出现棕黄色物质为阳性表达(+),无棕黄色物质为阴性表达(-)。
1.2.3 随访 通过书信及电话进行随访,预后观察起始点为:开腹手术日期,观察终点为死亡日期或本研究最后随访时间(2016年10月)。
1.3 统计学处理
采用SPSS 19.0对实验数据进行统计分析。采用四格表资料的χ2检验或Fisher确切概率法,Wilcoxon秩和检验,双变量Spearman's相关分析,Kaplan-Meier法绘制生存曲线及Log-rank检验对术后生存率分析,采用Cox回归模型进行多因素生存分析。生存时间以月计算,自手术当天起开始计算生存时间,患者死亡或失访定为随访结束点。检验结果取双侧,检验水准α=0.05,P<0.05为差异有统计学意义。
2 结 果
2.1 Maspin蛋白与p53蛋白在胆管癌组织中的表达
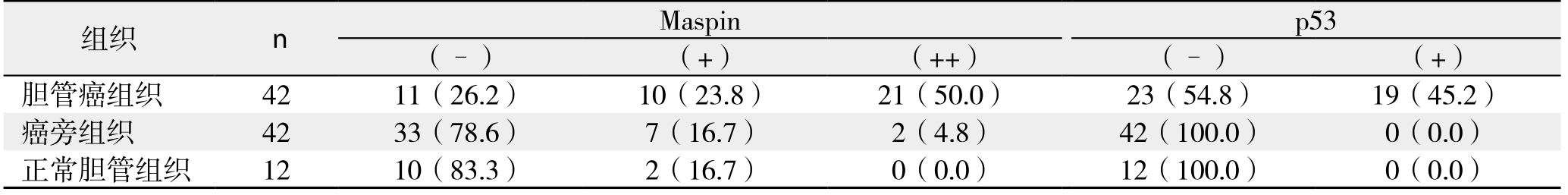
在42例原发性胆管癌组织中Maspin蛋白阳性率为73.8%,42例胆管癌旁组织和12例正常胆管组织Maspin蛋白阳性率分别为21.4%和16.7%,Maspin蛋白阳性率明显高于癌旁组(χ2=23.1,P=0.000)和正常胆管组(χ2=10.5,P=0.01);在42例原发性胆管癌组织p53蛋白阳性率为45.2%,在42例胆管癌旁组织和12例正常胆管组织p53蛋白阳性率均为0,差异均有统计学意义(χ2=22.0,P=0.000;χ2=6.5,P=0.01)(图1)(表1)。
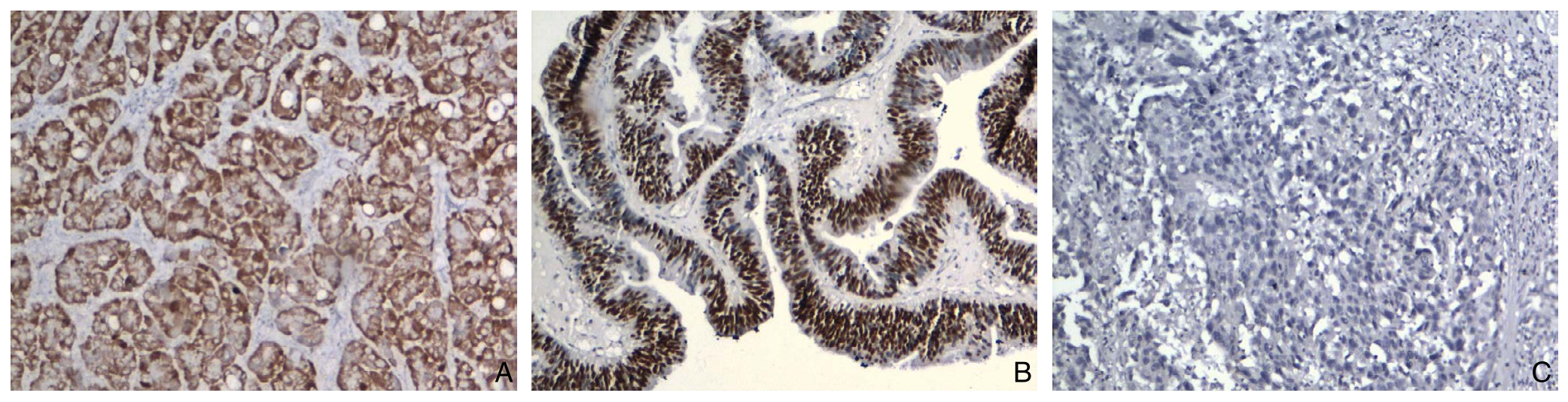
图1 免疫组化检测结果(×100) A:胆管癌组织Maspin阳性表达;B:胆管癌组织p53阳性表达;C:胆管癌组织Maspin阴性表达
Figure 1 Immunohistochemical staining findings A: Positive Maspin expression in cholangiocarinoma tissue; B: Positive p53 expression in cholangiocarinoma tissue; B: Negative Maspin expression in cholangiocarinoma tissue
表1 Maspin蛋白与p53蛋白在胆管癌、癌旁组织和正常胆管组织中的表达情况[n(%)]
Table 1 The expressions of Maspin and p53 protein in cholangiocarinoma, tumor adjacent tissue and normal biliary duct tissue [n (%)]
组织 n Maspin p53(–) (+) (++) (–) (+)胆管癌组织 42 11(26.2) 10(23.8) 21(50.0) 23(54.8) 19(45.2)癌旁组织 42 33(78.6) 7(16.7) 2(4.8) 42(100.0) 0(0.0)正常胆管组织 12 10(83.3) 2(16.7) 0(0.0) 12(100.0) 0(0.0)
2.2 Maspin与p53蛋白表达与胆管癌临床病理特征的相关性分析
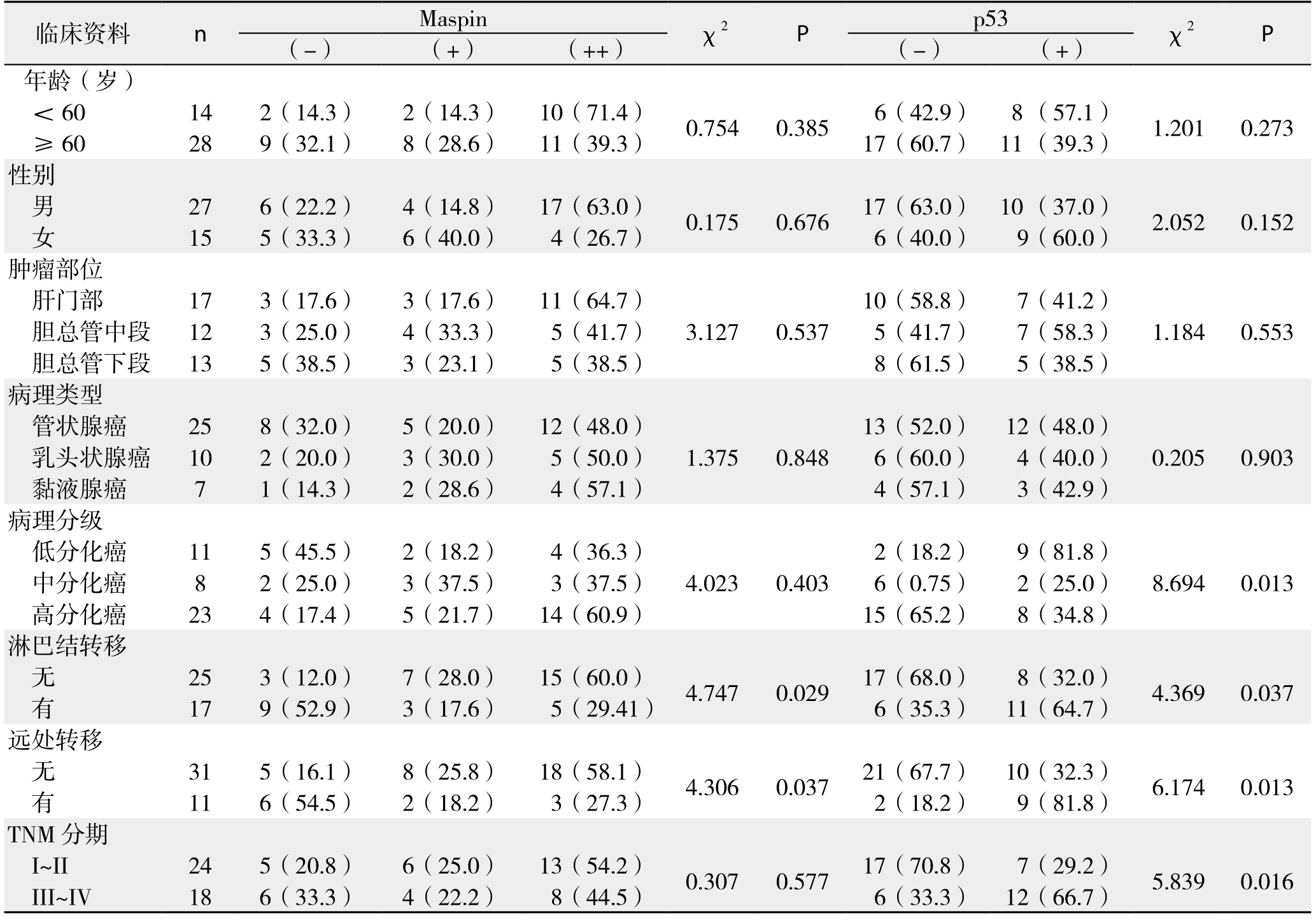
Maspin蛋白表达与胆管癌淋巴结转移(χ2=4.747,P=0.029)以及远处转移(χ2=4.306,P=0.037)有关,与年龄、性别、肿瘤部位、病理类型、病理分级以及TNM分期无关(均P>0.05);p53蛋白表达与胆管癌病理分级、TNM分期、淋巴结转移以及远处转移有关(均P<0.05),与年龄、性别、病理类型、肿瘤部位无关(均P>0.05)(表2)。
表2 Maspin、p53表达与患者临床病理特征的关系[n(%)]
Table 2 Relations of Maspin and p53 expression with cliniopathologic features the patients [n (%)]
临床资料 n Maspin χ2 P p53 χ2 P(-) (+) (++) (-) (+)年龄(岁)<60 14 2(14.3) 2(14.3) 10(71.4) 0.754 0.385 6(42.9) 8 (57.1) 1.201 0.273≥60 28 9(32.1) 8(28.6) 11(39.3) 17(60.7) 11 (39.3)性别男27 6(22.2) 4(14.8) 17(63.0) 0.175 0.676 17(63.0) 10 (37.0) 2.052 0.152女15 5(33.3) 6(40.0) 4(26.7) 6(40.0) 9(60.0)肿瘤部位肝门部 17 3(17.6) 3(17.6) 11(64.7) 10(58.8) 7(41.2)胆总管中段 12 3(25.0) 4(33.3) 5(41.7) 3.127 0.537 5(41.7) 7(58.3) 1.184 0.553胆总管下段 13 5(38.5) 3(23.1) 5(38.5) 8(61.5) 5(38.5)病理类型管状腺癌 25 8(32.0) 5(20.0) 12(48.0) 13(52.0) 12(48.0)乳头状腺癌 10 2(20.0) 3(30.0) 5(50.0) 1.375 0.848 6(60.0) 4(40.0) 0.205 0.903黏液腺癌 7 1(14.3) 2(28.6) 4(57.1) 4(57.1) 3(42.9)病理分级低分化癌 11 5(45.5) 2(18.2) 4(36.3) 2(18.2) 9(81.8)中分化癌 8 2(25.0) 3(37.5) 3(37.5) 4.023 0.403 6(0.75) 2(25.0) 8.694 0.013高分化癌 23 4(17.4) 5(21.7) 14(60.9) 15(65.2) 8(34.8)淋巴结转移无25 3(12.0) 7(28.0) 15(60.0) 4.747 0.029 17(68.0) 8(32.0) 4.369 0.037有17 9(52.9) 3(17.6) 5(29.41) 6(35.3) 11(64.7)远处转移无31 5(16.1) 8(25.8) 18(58.1) 4.306 0.037 21(67.7) 10(32.3) 6.174 0.013有11 6(54.5) 2(18.2) 3(27.3) 2(18.2) 9(81.8)TNM分期I~II 24 5(20.8) 6(25.0) 13(54.2) 0.307 0.577 17(70.8) 7(29.2) 5.839 0.016 III~IV 18 6(33.3) 4(22.2) 8(44.5) 6(33.3) 12(66.7)
2.3 胆管癌组中Maspin与p53蛋白表达之间的相关性
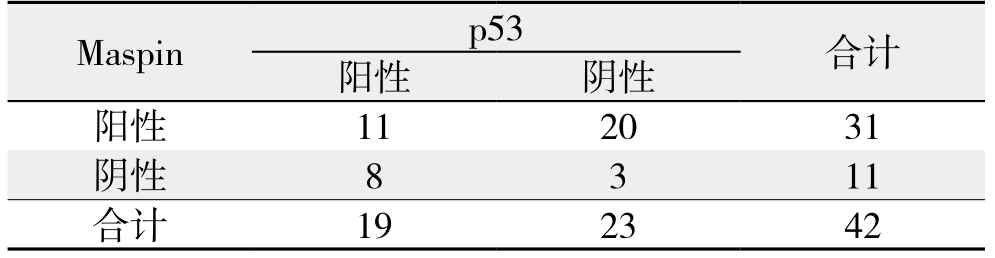
通过双变量Spearman's相关分析法可知,Maspin与p53蛋白表达无相关性(r=-0.329,P=0.144)(表3)。
表3 胆管癌组织中Maspin与p53蛋白表达之间的相关性分析(n)
Table 3 Correlation between Maspin and p53 protein expressions
in cholangiocarinoma tissue (n)
Maspin p53 合计阳性 阴性阳性 11 20 31阴性 8 3 11合计 19 23 42
2.4 Maspin及p53蛋白表达与胆管癌术后生存时间的关系
42例胆管癌患者获得随访患者有38例,失访4例,随访率90.5%。Maspin蛋白阳性与Maspin蛋白阴性的患者术后生存时间分别为(18.7±3.1)个月与(8.4±1.5)个月;术后中位生存时间分别为13个月与8个月。p53阳性与p53阴性的患者术后平均生存时间分别为(9.3±1.2)个月与(22.0±4.0)个月;术后中位生存时间分别为8个月与15个月。
采用Kaplan-Meier法绘制生存曲线(图2)及生存曲线Log-rank检验显示,Maspin阳性表达与阴性表达的患者术后生存时间之间的差别有统计学意义(χ2=4.440,P=0.035),Maspin阳性表达患者较阴性表达患者术后生存期延长。p53阳性表达与阴性表达的患者术后生存时间之间的差异有统计学意义(χ2=8.231,P=0.004),p53阴性表达患者较阳性表达患者术后生存期延长。
单因素总体生存率分析结果表明TNM分期、淋巴结转移、远处转移以及Maspin表达可预测胆管癌患者预后(均P<0.05)(表4);多因素Cox回归模型分析结果表明淋巴结转移、远处转移以及Maspin表达是胆管癌患者预后的独立影响因素(均P<0.05)(表5)。

图2 不同Maspin和p53状态胆管癌患者的生存曲线
Figure 2 Survival curves of cholangiocarinoma patients with different statuses of Maspin and p53 expression
表4 单因素总体生存率分析
Table 4 Univariate analysis of factors for overall survival rates
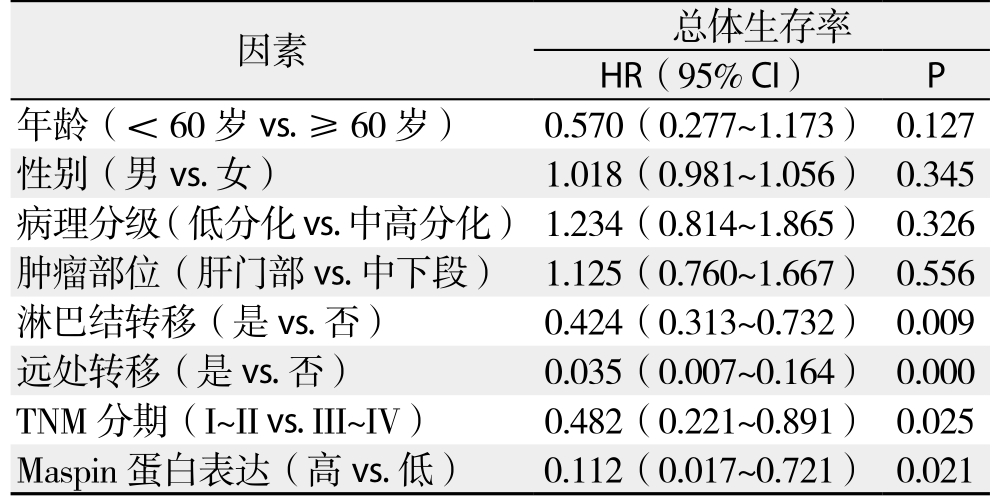
因素 总体生存率HR(95% CI) P年龄(<60岁vs.≥60岁) 0.570(0.277~1.173) 0.127性别(男vs.女) 1.018(0.981~1.056) 0.345病理分级(低分化vs.中高分化) 1.234(0.814~1.865) 0.326肿瘤部位(肝门部vs.中下段) 1.125(0.760~1.667) 0.556淋巴结转移(是vs.否) 0.424(0.313~0.732) 0.009远处转移(是vs.否) 0.035(0.007~0.164) 0.000 TNM 分期(I~II vs. III~IV) 0.482(0.221~0.891) 0.025 Maspin蛋白表达(高vs.低) 0.112(0.017~0.721) 0.021
表5 多因素Cox回归模型分析结果
Table 5 Analysis results of multivariate Cox regression model
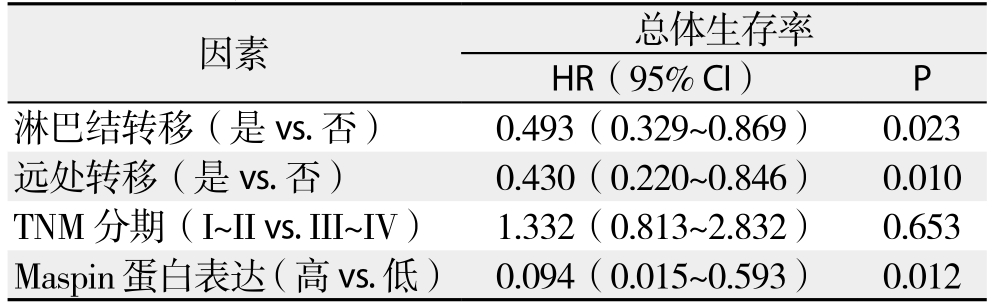
因素 总体生存率HR(95% CI) P淋巴结转移(是vs.否) 0.493(0.329~0.869) 0.023远处转移(是vs.否) 0.430(0.220~0.846) 0.010 TNM分期(I~II vs. III~IV) 1.332(0.813~2.832) 0.653 Maspin蛋白表达(高vs.低) 0.094(0.015~0.593) 0.012
3 讨 论
Maspin属于丝氨酸家族的一种新型丝氨酸蛋白酶抑制剂, 它归属于SERPIN超家族,是Zon等[8]于1994年采用杂交消减技术于正常乳腺上皮组织中发现的。与正常的乳腺组织相比, Maspin在相应的乳腺癌中表达明显下降, 其功能相当于抑癌基因[9]。但在最近研究[10-11]发现与之相反的结论,在胃癌与子宫内膜腺癌中频繁检测到Maspin的反常表达。笔者[12]在前期研究中同样发现,在甲状腺癌中Maspin蛋白阳性率高达48.5%(32/66),而在甲状腺良性病变和正常甲状腺组织均不表达。Xu等[13]论证这种反常的表达可能与Maspin基因5'端调节区的脱甲基相关,他们证实,Maspin蛋白是通过对CpG岛(CpG岛是由CpG二核苷酸序列聚集而成)脱甲基作用来抑制细胞裂解,从而改变Maspin的表达。
最近Kanzawa等[5]报道,Maspin在胆管癌中表达增强:良性增生上皮(20%)、胆道上皮内瘤(<50%)、侵入性胆管癌(>90%)。本研究也发现了这种反常表达,与正常胆管组织及癌旁组织相比,Maspin蛋白在胆管癌中高表达。这种反常表达与乳腺癌[9,14]、前列腺癌[15]和口腔癌[16]中表达完全相反。在胆管癌致癌机理中,Maspin可能充当一个异于寻常的角色,在胆管癌变一系列过程中,Maspin的表达可能具有独有的特性,可以推断胆管癌变过程与其他类型的肿瘤可能不同。本研究示,Maspin蛋白在胆管癌中表达升高,提示了Maspin蛋白可能是胆管癌肿瘤标志物之一,这在鉴别胆管癌有一定价值。
Dabiri等[9,17]研究发现,在乳腺癌细胞中Maspin蛋白可以通过增加细胞的黏附能力和减低细胞的运动能力来抑制肿瘤浸润。Amir等[18]研究结果表明,Maspin能抑制前列腺癌细胞表面的尿激酶纤维蛋白溶酶原激活物(uPA),uPA能降低肿瘤细胞的浸润。Dzinic等[19]研究结果也表明,Maspin蛋白能抑制前列腺癌细胞的复发与扩散。最新研究报道,在恶性黑素瘤[20]及皮肤鳞癌[21]中,Maspin表达缺失可导致肿瘤转移与扩散,同样佐证了Maspin在恶性黑素瘤及皮肤鳞癌中具有肿瘤抑制作用。在本研究中,Maspin蛋白表达与胆管癌淋巴结转移以及远处转移有关,随着胆管癌细胞的侵袭性加剧,而Maspin蛋白表达反而下调,提示了Maspin在胆管癌中同样具有肿瘤抑制功能。
Chen等[22]最新研究结果表明,在肝细胞癌中Maspin低表达患者的总体生存率明显下降。本研究中,采用Kaplan-Meier法绘制生存曲线及生存曲线Log-rank检验显示,Maspin阳性表达患者术后生存期延长,单因素分析和多因素Cox回归模型分析结果也表明,Maspin表达是胆管癌患者预后的独立影响因素,Maspin蛋白表达可作为预测胆管癌患者预后的指标。
目前更精确的Maspin蛋白的细胞学活性和生物化学活性尚不清楚[23]。Celik等[24]最近研究发现,在睾丸肿瘤中,p53表达可能影响Maspin表达,p53与Maspin表达正相关。而Choy等[25]研究发现,在肺鳞癌中,p53能激活Maspin表达的启动子,p53与Maspin表达存在着正相关,但在肺腺癌中,p53与Maspin表达无相关。本研究结果也显示胆管癌组织中Maspin蛋白与p53蛋白表达无相关性。这可能与样本量的大小可能有关。
综上所述,通过本研究发现,Maspin蛋白在胆管癌组织中表达增加, 提示了Maspin蛋白可能是胆管癌肿瘤标志物之一,其表达减少或缺失与患者不良临床病理特征及预后密切相关,Maspin蛋白有可能作为预测胆管癌患者预后因子,并可望作为生物治疗的作用靶点。但Maspin蛋白更精确的作用机制还有待我们更进一步探索。
参考文献
[1] 周少君, 黄志勇. 肝内胆管癌根治性切除术后肿瘤复发转移的预后因素分析[J]. 中国普通外科杂志, 2014, 23(8):1024–1029.doi:10.7659/j.issn.1005–6947.2014.08.002.Zhou SJ, Huang ZY. Prognostic factors for tumor recurrence and metastasis of intrahepatic cholangiocarcinoma after radical resection[J]. Chinese Journal of General Surgery, 2014, 23(8):1024–1029. doi:10.7659/j.issn.1005–6947.2014.08.002.
[2] Gera S, Ettel M, Acosta-Gonzalez G, et al. Clinical features,histology, and histogenesis of combined hepatocellularcholangiocarcinoma[J]. World J Hepatol, 2017, 9(6):300–309. doi:10.4254/wjh.v9.i6.300.
[3] Vogel A, Saborowski A. Cholangiocellular Carcinoma[J]. Digestion,2017, 95(3):181–185. doi: 10.1159/000454763.
[4] Maroni L, Pierantonelli I, Banales JM, 等. 遗传学在胆管细胞癌发展中的重要性[J]. 中国普通外科杂志, 2015, 24(2):151–162.doi:10.3978/j.issn.1005–6947.2015.02.001.Maroni L, Pierantonelli I, Banales JM, et al. The significance of genetics for cholangiocarcinoma development[J]. Chinese Journal of General Surgery, 2015, 24(2):151–162. doi:10.3978/j.issn.1005–6947.2015.02.001.
[5] Kanzawa M, Sanuki T, Onodera M, et al. Double immunostaining for maspin and p53 on cell blocks increases the diagnostic value of biliary brushing cytology[J]. Pathol Int, 2017, 67(2):91–98. doi:10.1111/pin.12505.
[6] Jenkinson SE, Brown LJ, Ombor J, et al. Identification of novel peptide motifs in the serpin maspin that affect vascular smooth muscle cell function[J]. Biochim Biophys Acta, 2017, 1864(2):336–344. doi: 10.1016/j.bbamcr.2016.11.019.
[7] Sun P, Wu Q, Ruan G, et al. Expression patterns of maspin and mutant p53 are associated with the development of gestational trophoblastic neoplasia[J]. Oncol Lett, 2016, 12(5):3135–3142.
[8] Zon Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells[J].Science, 1994, 263(5146):526–529.
[9] Dabiri S, Moeini Aqhtaei M, Shahryari J, et al. Maspin Gene Expression in Invasive Ductal Carcinoma of Breast [J]. Iran J Pahthol, 2016, 11(2):104–111.
[10] Stella B, Lara A, Carlo S, et al. Maspin expression, subcellular localization and clinicopathological correlation in endometrial hyperplasia and endometrial adenocarcinoma[J]. Histol Histopathol,2014, 29(6):777–783. doi: 10.14670/HH–29.777.
[11] Taskiran C, Erdem O, Onan A, et al. Maspin expression in endometrial hyperplasia and carcinoma, and its relation with angiogenesis[J]. Eur J Gynaecol Oncol, 2014, 35(2):134–139.
[12] 王逸君, 王志明, 李新营, 等. Maspin在甲状腺癌中的表达及其临床意义[J]. 中国普通外科杂志, 2009, 18(5):515–517.Wang YJ, Wang ZM, Li XY, et al. Expressions and dinical significance of Maspin in thyroid carcinoma[J]. Chinese Journal of General Surgery, 2009, 18(5):515–517.
[13] Xu L, Liu H, Yu J, et al. Methylation-induced silencing of maspin contributes to the proliferation of human glioma cells[J].Oncol Rep,2016, 36(1):57–64. doi: 10.3892/or.2016.4783.
[14] Bernardo MM, Dzinic SH, Matta MJ, et al. The Opportunity of Precision Medicine for Breast Cancer With Context-Sensitive Tumor Suppressor Maspin[J]. J Cell Biochem, 2017, 118(7):1639–1647. doi: 10.1002/jcb.25969.
[15] Wu CT, Wang WC, Chen MF, et al. Glucose-regulated protein 78 mediates hormone-independent prostate cancer progression and metastasis through maspin and COX-2 expression[J]. Tumour Biol,2014, 35(1):195–204. doi: 10.1007/s13277–013–1024–4.
[16] Choi KY, Choi HJ, Chung EJ, et al. Loss of heterozygosity in mammary serine protease inhibitor (maspin) and p53 at chromosome 17 and 18 in oral cavity squamous cell carcinoma[J].Head Neck, 2015, 37(9):1239–1245. doi: 10.1002/hed.23741.
[17] Strien L, Joensuu K, Heikkilä P, et al. Different Expression Patterns of CXCR4, CCR7, Maspin and FOXP3 in Luminal Breast Cancers and Their Sentinel Node Metastases[J]. Anticancer Res, 2017,37(1):175–182.
[18] Amir S, Margaryan NV, Odero-Marah V, et al. Maspin regulates hypoxia-mediated stimulation of uPA/uPAR complex in invasive breast cancer cells[J]. Cancer Biol Ther, 2005, 4(4):400–406.
[19] Dzinic SH, Chen K, Thakur A, et al. Maspin expression in prostate tumor elicits host anti-tumor immunity[J]. Oncotarget, 2014, 5(22):11225–11236.
[20] Ribero S, Senetta R, Osella-Abate S, et al. Prognostic role of maspin expression in melanoma: probably far from clinical use[J].Histopathology, 2017, 71(1):158–162. doi: 10.1111/his.13188.
[21] Zhu H, Mao Q, Liu W, et al. Maspin suppresses growth,proliferation and invasion in cutaneous squamous cell carcinoma cells[J]. Oncol Rep, 2017, 37(5):2875–2882. doi: 10.3892/or.2017.5574.
[22] Chen WS, Yen CJ, Chen YJ, et al. miRNA-7/21/107 contribute to HBx-induced hepatocellular carcinoma progression through suppression of maspin[J]. Oncotarget, 2015, 6(28): 25962–25974.doi: 10.18632/oncotarget.4504.
[23] Dzinic SH, Bernardo MM, Oliveira DS, et al. Tumor suppressor maspin as a modulator of host immune response to cancer[J]. Bosn J Basic Med Sci, 2015, 15(4):1–6. doi: 10.17305/bjbms.2015.783.
[24] Celik H, Turunc T, Bal N, et al. Expression of maspin in testis tumors with germ cells andits relation with angiogenesis factors[J].Turk J Med Sci, 2016, 46(4):1197–1202. doi: 10.3906/sag–1504–134.
[25] Choy B, Findeis-Hosey JJ, Li F, et al. High frequency of coexpression of maspin with p63 and p53 in squamous cell carcinoma but not in adenocarcinoma of the lung [J]. Int J Chin Exp pathol, 2013, 6(11): 2542–2547.