胰十二指肠切除术(pancreaticoduodenectomy,PD)是胰头和壶腹周围肿瘤的标准术式,也是普通外科中难度最大的手术之一。此类患者常有低蛋白血症、梗阻性黄疸等合并症,并且PD术涉及器官多、操作复杂、手术时间长、机体应激强烈,给麻醉管理带来挑战,各种不良反应如苏醒延迟、恶心、呕吐、腹胀、术后疼痛等亦较多见。超声引导下的椎旁阻滞具有操作简便、安全可靠等优势,受到麻醉医师关注。本研究目的在于观察超声引导椎旁阻滞在PD术中应用的镇痛效果,并观察术后不良反应的发生率。
1 资料与方法
1.1 一般资料
选取2016年4月—2016年12月在我院行择期开腹PD术的43例患者,按照随机数字表法分为对照组(21例)和观察组(22例),对照组行单纯全身麻醉,观察组行全身麻醉复合椎旁阻滞。排除标准:严重器官功能障碍者;姑息手术或急诊手术者;患者不配合或合并精神症状者;穿刺节段有严重脊柱畸形或实施过手术者;穿刺部位感染者;凝血功能障碍者。退出标准:呼吸抑制[呼吸频率(RR)<8次/min或吸氧时血氧饱和度(SpO2)<90%];患者处于不能耐受或危及生命的状况(如术后48 h内再次手术、肺栓塞等)。本研究已获本院医学伦理委员会批准,并与患者签署知情同意书。
1.2 麻醉方法
患者入室后常规吸氧,开放外周静脉,监测SpO2、心电图(ECG)、心率(HR)和血压(BP)等,行桡动脉置管直接测压。对照组行常规全身麻醉,诱导给予咪达唑仑0.05 mg/kg,舒芬太尼0.5 µg/kg、苯磺顺阿曲库铵0.15 mg/kg和丙泊酚0.5~1 mg/kg。插管完成后连接麻醉机行间歇正压通气,调节VT 6~10 mL/kg,RR 10~12次/min,I:E为1:2,维持PETCO2为35~45 mmHg(1 mmHg=0.133 kPa)。麻醉维持采用靶控输注丙泊酚,脑电双频指数(BIS)监测麻醉深度,调节靶控浓度维持BIS值40~60。间断静推苯磺顺阿曲库铵维持肌松。切皮前不追加镇痛药物,切皮后根据患者BP和HR变化追加舒芬太尼镇痛,维持循环波动在基础值20%内。观察组于全身麻醉诱导前于T8/T9、T11/T12间隙行椎旁神经阻滞。椎旁神经阻滞方法为患者取左侧卧位,常规消毒铺巾,使用Sonosite M-Turbo超声仪,通过“12肋出现法”定位胸椎节段和横突后,超声探头与脊柱平行,采用平面外法进针,当针尖突破肋横突上韧带后,试推生理盐水导致胸膜下移,回抽无血、无气后每个间隙注射0.4%罗哌卡因10 mL。20min后,通过针刺痛检测阻滞平面,判断阻滞效果。确认阻滞完善后,行全身麻醉,诱导和维持方案同对照组。两组术后均采用患者自控静脉镇痛(PCIA),方案为2 µg/kg舒芬太尼稀释至100 mL,背景量2 mL/h,单次剂量0.5 mL,锁定时间15min。术后不给予其他补救药物。
1.3 观察指标
⑴ 记录入室平静后、切皮前、切皮后5min、手术结束前患者平均动脉压(MAP)和HR,记录术中舒芬太尼用量;⑵ 记录术后2、16、24、48 h的安静和90°翻身时的视觉模拟评分(VAS,0分为无痛,10分代表难以忍受的剧烈疼痛);⑶ 记录术后48 h时镇痛泵按压次数,术后患者恶心、呕吐、出汗、眩晕、瘙痒、谵妄、呼吸抑制等不良反应的发生率。
1.4 统计学处理
采用SPSS 19.0统计软件进行统计分析。其中计量资料采用均数±标准差( ±s)表示,组间比较采用t检验,计数资料采用χ2检验,P<0.05为差异有统计学意义。
±s)表示,组间比较采用t检验,计数资料采用χ2检验,P<0.05为差异有统计学意义。
2 结 果
2.1 两组患者术前资料比较
对照组和观察组两组患者性别、年龄、体质量、ASA分级、术前VAS等基线数据比较差异无统计学意义(均P>0.05)(表1)。
2.2 两组镇痛效果及其他相关指标比较
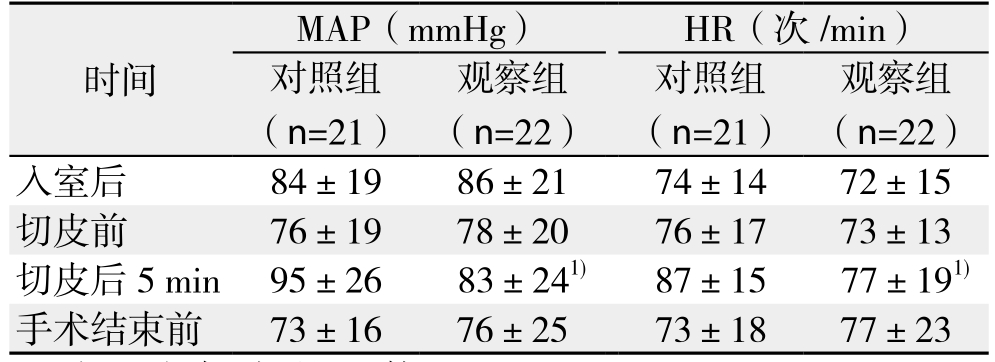
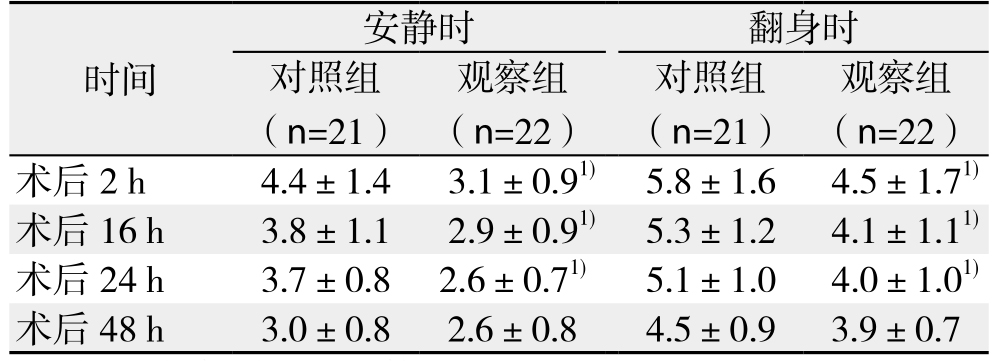
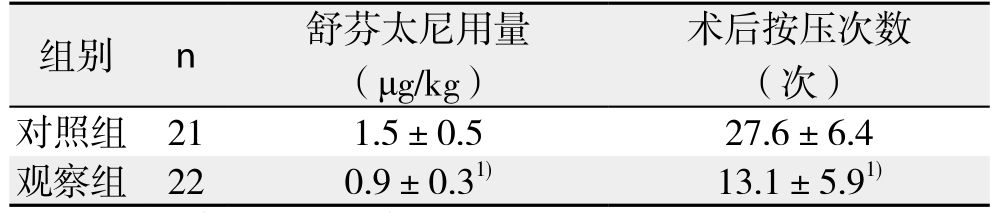
切皮后5min观察组MAP和HR明显低于对照组(均P<0.05),入室平静后、切皮前和手术结束前3个时间点无统计学差异(均P>0.05)(表2)。术后2、16、24 h时观察组安静和90°翻身VAS评分均明显低于对照组(均P<0.05);48 h时两组VAS评分接近(P>0.05),此时为轻-中度疼痛,无需补救措施能入睡(表3)。术中舒芬太尼用量、术后PCIA按压次数观察组明显低于对照组(均P<0.05)(表4)。
表1 两组患者的基线数据比较
Table 1 Comparison of the baseline data in two groups
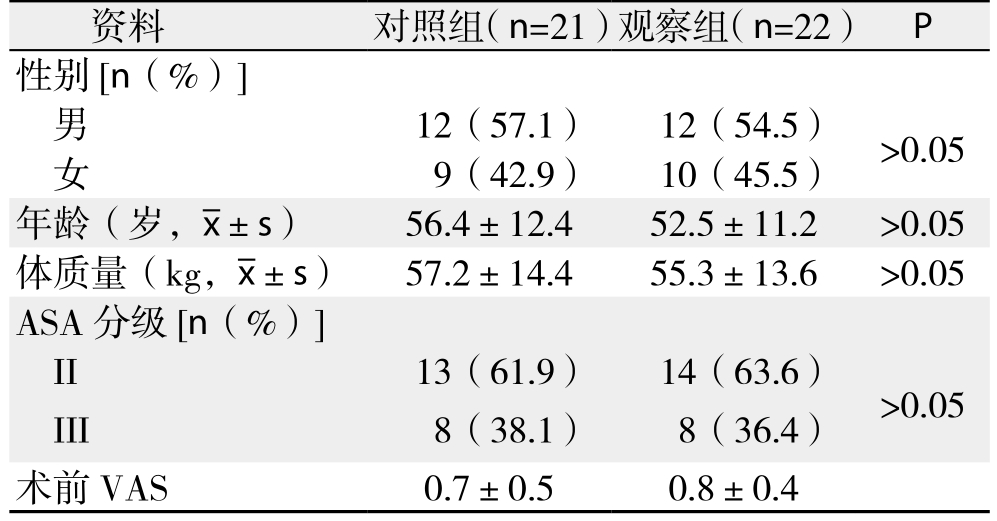
资料 对照组(n=21)观察组(n=22) P性别[n(%)]男12(57.1) 12(54.5) >0.05女9(42.9) 10(45.5)年龄(岁, ±s) 56.4±12.4 52.5±11.2 >0.05体质量(kg,
±s) 56.4±12.4 52.5±11.2 >0.05体质量(kg, ±s) 57.2±14.4 55.3±13.6 >0.05 ASA分级[n(%)]II 13(61.9) 14(63.6) >0.05 III 8(38.1) 8(36.4)术前VAS 0.7±0.5 0.8±0.4
±s) 57.2±14.4 55.3±13.6 >0.05 ASA分级[n(%)]II 13(61.9) 14(63.6) >0.05 III 8(38.1) 8(36.4)术前VAS 0.7±0.5 0.8±0.4
表2 两组患者围术期MAP和HR的比较( ±s)
±s)
Table 2 Comparison of MAP and HR between the two groups( ±s)
±s)
注:1)与对照组比较,P<0.05
Note: 1) P<0.05 vs. control group
观察组(n=22)入室后 84±19 86±21 74±14 72±15切皮前 76±19 78±20 76±17 73±13切皮后 5min 95±26 83±241) 87±15 77±191)手术结束前 73±16 76±25 73±18 77±23时间MAP(mmHg) HR(次 /min)对照组(n=21)观察组(n=22)对照组(n=21)
表3 两组患者术后不同时间点不同状态下VAS评分( ±s)
±s)
Table 3 Comparison of VAS at different time points and states between the two groups ( ±s)
±s)
注:1)与对照组比较,P<0.05
Note: 1) P<0.05 vs. control group
时间 观察组(n=22)术后 2 h 4.4±1.4 3.1±0.91) 5.8±1.6 4.5±1.71)术后 16 h 3.8±1.1 2.9±0.91) 5.3±1.2 4.1±1.11)术后 24 h 3.7±0.8 2.6±0.71) 5.1±1.0 4.0±1.01)术后 48 h 3.0±0.8 2.6±0.8 4.5±0.9 3.9±0.7安静时 翻身时对照组(n=21)观察组(n=22)对照组(n=21)
表4 两组患者术中舒芬太尼用量、术后PCIA按压次数( ±s)
±s)
Table 4 Comparison of sulfentanil consumption amount and PCIA requirements between the two groups ( ±s)
±s)
注:1)与对照组比较,P<0.05
Note: 1) P<0.05 vs. control group
组别 n 舒芬太尼用量(μg/kg)术后按压次数(次)对照组 21 1.5±0.5 27.6±6.4观察组 22 0.9±0.31) 13.1±5.91)
2.3 两组患者不良反应发生情况比较
观察组恶心、呕吐、出汗、眩晕、瘙痒、谵妄等不良反应的发生率与对照组的差异均无统计学意义(均P>0.05),两组均未发生呼吸抑制(表5)。
表5 两组患者术后不良反应的比较[n(%)]
Table 5 Comparison of adverse effects after operation between the two groups [n (%)]

组别 n 恶心 呕吐 出汗 眩晕 瘙痒 谵妄 呼吸抑制对照组 21 8(38.1) 7(33.3) 3(14.3) 5(23.8) 3(14.3) 5(23.8) 0(0.0)观察组 22 5(22.7) 5(22.7) 1(4.5) 3(13.6) 2(9.1) 2(9.1) 0(0.0)
3 讨 论
胸段椎旁间隙是位于椎体旁的一个上下相通的楔形间隙,由肋横突韧带、壁层胸膜、椎体、椎间孔和椎间盘外侧围成,向外与肋间隙相通,向内与椎管相通,有肋间神经、脊神经后支、灰白交通支和交感链通过[1]。在一个节段给予局麻药,能够剂量依赖性的上下扩散几个节段。与硬膜外阻滞相比,仅产生单侧阻滞的效果,因此对呼吸和循环干扰较小[2]。传统的椎旁阻滞方法采用盲法穿刺,在确定节段的棘突旁开3.0 cm,垂直皮肤进针触及横突,调整方向,突破肋横突韧带时有突破感,凭体表标志和手感实施阻滞,有一定的阻滞失败率和并发症的发生[3]。本研究采用超声引导旁矢状位平面外进针技术,首先采用“12肋出现法”快速精确定位椎体节段,识别横突和胸膜后,将局麻药注射于肋横突韧带以下,可见药液将胸膜向下推移,上下节段胸膜亦被推移。根据超声下肋横突韧带深度、超声下组织运动、药物扩散、突破感决定穿刺深度和方向,与平面内技术比,具有穿刺方向不朝向椎间孔、无横突阻挡、路径短、一个界面可见多个节段胸膜等优点[4]。前期研究发现,胸椎旁单点注药后,药物的扩散具有不确定性,因此本研究采用多点注射实现稳定的多节段阻滞。这与Kasimahanti等[5]在乳房切除术对比单点和双点椎旁阻滞的研究结论一致。椎旁持续输注的镇痛效果优于单次注射,但有研究发现椎旁阻滞容易实施但置管失败率较高[6],因此本研究采用单次双点椎旁阻滞复合PCIA的镇痛模式。
目前椎旁阻滞在胸科手术多模式镇痛中的应用已有充分的证据证实其效果,研究[7]显示,在胸科手术中椎旁阻滞与硬膜外阻滞同样有效,而并发症发生率更低(尿储留,恶心、瘙痒、低血压、呼吸抑制、肺部并发症)。椎旁阻滞在腹部手术中的应用尚未得到公认,临床应用较少。有研究[8]表明腹横肌平面阻滞(TAPB)和胸段椎旁阻滞均能安全有效的应用于上腹部手术,椎旁阻滞组的VAS评分和第1次追加镇痛药时间更长,患者镇痛需求和术后恶心呕吐率更低。Schreiber等[9]在开腹肝叶切除术中对比硬膜外阻滞和双侧椎旁阻滞的镇痛效果,发现硬膜外组镇痛优势有限。EI-Boghdadly等[10]针对椎旁阻滞在腹部手术的应用做了综述,纳入20个随机对照临床试验的1044例患者,发现与全身性给药镇痛相比,疼痛评分与阿片药的消耗均有改善,但尚无充分证据证明其比硬膜外阻滞或其他外周神经阻滞更有优势,需要更好、更充分的研究来证明这一点。传统观点认为腰大肌将胸段和腰段椎旁间隙隔开,而有研究显示在胸段椎旁间隙单次注药后,药物能往腰段椎旁间隙扩散[11],在腰段产生镇痛效果。
PD术涉及胰腺、十二指肠、胆道、胃和空肠等脏器,涉及解剖层面多,操作复杂[12-13],操作时间长,应激强烈,单纯全身麻醉常需使用较大剂量的阿片药镇痛来抑制应激反应。而大剂量阿片药易导致术后腹胀、胃肠蠕动慢、恶心呕吐、呼吸抑制等并发症[14]。加速康复外科的理念是积极促进术后器官功能的快速康复[15-16],其中肠道功能是非常重要的一环,直接影响到患者的住院时间,所以加速康复外科要求在镇痛完善的前提下尽量少用阿片药。
开腹PD术中术后疼痛剧烈[17],主要可以分为切口痛、内脏痛和炎性痛,PD术的切口较长,呈J型,经由腹中线,脐水平向右侧至腋前线,主要位于T6至T10平面。而内脏受交感神经和副交感神经的双重支配,交感神经支的阻滞平面应达到脊髓T4至L1节段,而此平面范围内迷走神经支不可能被阻滞。本研究椎旁阻滞后20min右侧躯干平面能达到T6-7至T12、L1,因此能很大程度上缓解PD术中疼痛和牵拉应激。椎旁阻滞仅阻滞单侧交感神经,因此较少造成循环不稳定。本研究结果表明,胸段椎旁阻滞能够明显提高术中和术后24 h内镇痛效果,减少阿片药物的消耗量,超声引导下无1例穿刺相关并发症的发生。24 h以后,随着椎旁阻滞效果的消退,两组疼痛评分趋于接近,此时安静时基本上为轻度疼痛,不太影响睡眠。尽管观察组发生术后不良反应的患者数多于对照组,但差异无统计学意义,可能与样本量较少或单次椎旁阻滞时间有限有关。
加速康复外科的理念最早应用于结直肠手术取得了良好结果,在PD术中应用后同样为患者带来了益处[18-19],中国学者[22]在此领域也进行了积极的探索,取得了一些成绩[20-21]。加速康复理念中非常重要的一点就是加强围术期镇痛。术后硬膜外镇痛能提供很好的镇痛效果,这也写进了各种加速康复外科指南[23-24],但其固有的一些问题如穿刺禁忌、穿刺失败、低血压、尿储留、影响下肢肌力、感染、导管移位、护理不便等始终未能解决。同时越来越多的证据表明,尽管有研究[25]显示硬膜外镇痛能降低心血管疾病和肺部并发症的发生率,但仅限于高危患者接受大型胸部和腹部手术或局麻患者接受胸椎硬膜外镇痛,微创区域镇痛技术和硬膜外技术一样行之有效,因此对于常规术后镇痛,硬膜外镇痛可能不再被认为是金标准。本研究提示单次椎旁阻滞结合静脉镇痛可提供良好的术中术后镇痛效果,可作为开腹PD术镇痛方案的选择之一,其降低术后不良反应发生率的可行性需要通过增加样本量或延长阻滞时间来进一步研究。
参考文献
[1]刘勇, 余虓, 王少刚, 等. 超声引导椎旁阻滞麻醉在经皮肾镜碎石术中的应用[J]. 临床麻醉学杂志, 2016, 32(11):1135–1136.Liu Y, Yu X, Wang SG, et al. Application of ultrasound-guided Paravertebral Block in Percutaneous nephrolithotomy[J]. Journal of Clinical Anesthesiology, 2016, 32(11):1135–1136.
[2]Cheng GS, Ilfeld BM. A review of postoperative analgesia for breast cancer surgery[J]. Pain Manag, 2016, 6(6):603–618.
[3]田杨, 许挺, 徐懋. 超声引导下胸椎旁阻滞的进展[J]. 中国微创外科杂志, 2016, 16(4):359–361. doi:10.3969/j.issn.1009–6604.2016.04.019.Tian Y, Xu T, Xu M. Progress on Ultrasound-guided Thoracic Paravertebral Block[J]. Chinese Journal of Minimally Invasive Surgery, 2016, 16(4):359–361. doi:10.3969/j.issn.1009–6604.2016.04.019.
[4]Amlong C, Guy M, Schroeder KM, et al. Out-of-plane ultrasoundguided paravertebral blocks improve analgesic outcomes in patients undergoing video-assisted thoracoscopic surgery[J]. Local Reg Anesth, 2015, 8:123–128. doi: 10.2147/LRA.S86853.
[5]Kasimahanti R, Arora S, Bhatia N, et al. Ultrasound-guided singlevs double-level thoracic paravertebral block for postoperative analgesia in total mastectomy with axillary clearance[J]. J Clin Anesth, 2016, 33:414–421. doi: 10.1016/j.jclinane.2016.01.027.
[6]Cowie B, McGlade D, Ivanusic J, et al. Ultrasound-guided thoracic paravertebral blockade: a cadaveric study[J]. Anesth Analg, 2010,110(6):1735–1739. doi: 10.1213/ANE.0b013e3181dd58b0.
[7]Scarci M, Joshi A, Attia R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management?[J]. Interact Cardiovasc Thorac Surg, 2010, 10(1):92–96. doi: 10.1510/icvts.2009.221127.
[8]Elsayeda RM, El Kareim ET. Comparative study between ultrasound guided tap block and paravertebral block in upper abdominal surgeries. Randomized controlled trial [J]. Egypt J Anesth, 2017,33(1):83–89. https://doi.org/10.1016/j.egja.2016.08.018.
[9]Schreiber KL, Chelly JE, Lang RS, et al. Epidural Versus Paravertebral nerve block for postoperative analgesia in patients undergoing open liver resection: a randomized clinical trial[J].Reg Anesth Pain Med, 2016, 41(4):460–468. doi: 10.1097/AAP.0000000000000422.
[10]EI-Boghdadly K, Madjdpour C, Chin KJ. Thoracic paravertebral blocks in abdominal surgery-a systematic review of randomized controlled trials[J]. Br J Anaesth, 2016, 117(3):297–308. doi:10.1093/bja/aew269.
[11]Albokrinov AA, Fesenko UA. Spread of dye after single thoracolumbar paravertebral injection in infants: a cadaveric study[J]. Eur J Anaesthesiol, 2014, 31(6):305–309. doi: 10.1097/EJA.0000000000000071.
[12]Doula C, Kostakis ID, Damaskos C, et al. Comparison between minimally invasive and open pancreaticoduodenectomy: a systematic review[J]. Surg Laparosc Endosc Percutan Tech, 2016,26(1):6–16. doi: 10.1097/SLE.0000000000000228.
[13]孙明生, 万波, 龚毅, 等. 腹腔镜胰十二指肠切除术相关肠系膜上血管应用解剖学研究[J]. 中国普通外科杂志, 2016, 25(3):394–400. doi:10.3978/j.issn.1005–6947.2016.03.015.Sun MS, Wan B, Gong Y, et al. Applied anatomy of superior mesenteric vessels associated with laparoscopic pancreaticoduodenectomy via uncinate process approach[J]Chinese Journal of General Surgery, 2016, 25(3):394–400. doi:10.3978/j.issn.1005–6947.2016.03.015.
[14]Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate Enhanced Recovery After Surgery pathways[J].Can J Anaesth,2015, 62(2):203–218. doi: 10.1007/s12630–014–0275–x.
[15]Ljungqvist O. ERAS--enhanced recovery after surgery:moving evidence-based perioperative care to practice[J].JPEN J Parenter Enteral Nutr, 2014, 38(5):559–566. doi:10.1177/0148607114523451.
[16]宋伟, 邹书兵. 加速康复外科在肝脏手术围手术期应用的Meta分析[J]. 中国普通外科杂志, 2016, 25(1):115–125. doi:10.3978/j.issn.1005–6947.2016.01.018.Song W, Zou SB. Application of enhanced recovery after surgery in setting of liver surgery: a Meta-analysis[J]. Chinese Journal of General Surgery, 2016, 25(1):115–125. doi:10.3978/j.issn.1005–6947.2016.01.018.
[17]Weber CE, Bock EA, Hurtuk MG, et al. Clinical and pathologic features influencing survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma[J]. J Gastrointest Surg, 2014, 18(2):340–347. doi: 10.1007/s11605–013–2388–x.
[18]林天生, 陈博滔, 孙维佳. 快速康复外科在胰十二指肠切除术围手术期的应用[J]. 中国普通外科杂志, 2015, 24(3):418–425.doi:10.3978/j.issn.1005–6947.2015.03.022.Lin TS, Chen BT, Sun WJ. Use of enhanced recovery in perioperative care of panceaticoduodenectomy[J]. Chinese Journal of General Surgery, 2015, 24(3):418–425. doi:10.3978/j.issn.1005–6947.2015.03.022.
[19]Ni TG, Yang HT, Zhang H, et al. Enhanced recovery after surgery programs in patients undergoing hepatectomy: A meta-analysis[J].World J Gastroenterol, 2015, 21(30):9209–9216. doi: 10.3748/wjg.v21.i30.9209.
[20]Deng X, Cheng X, Huo Z, et al. Modified protocol for enhanced recovery after surgery is beneficial for Chinese cancer patients undergoing pancreaticoduodenectomy[J]. Oncotarget, 2017,8(29):47841–47848. doi: 10.18632/oncotarget.18092.
[21]周永平, 戴途, 华志元, 等. 加速康复外科在胰十二指肠切除术的临床应用[J]. 中华肝胆外科杂志, 2017, 23(5):320–322.doi:10.3760/cma.j.issn.1007–8118.2017.05.010.Zhou YP, Dai T, Hua ZY, et al. Enhanced recovery after surgery in pancreaticoduodenectomy[J]. Chinese Journal of Hepatobiliary Surgery, 2017, 23(5):320–322. doi:10.3760/cma.j.issn.1007–8118.2017.05.010.
[22]欧阳剑波, 黄耿文, 何文, 等. 多学科合作快速康复外科理念在腹腔镜腹股沟疝修补术围手术期的应用[J]. 中国普通外科杂志,2017, 26(4):506–513. doi:10.3978/j.issn.1005–6947.2017.04.017.Ouyang JB, Huang GW, He W, et al. Application of multidisciplinary enhanced recovery after surgery in perioperative period of laparoscopic inguinal hernia repair[J]. Chinese Journal of General Surgery, 2017, 26(4):506–513. doi:10.3978/j.issn.1005–6947.2017.04.017.
[23]Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations[J].World J Surg, 2013,37(2):240–258. doi: 10.1007/s00268–012–1771–1.
[24]Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations[J]. Clin Nutr, 2012,31(6):817–830. doi: 10.1016/j.clnu.2012.08.011.Rawal N. Epidural technique for postoperative pain: gold standard no more?[J]. Reg Anesth Pain Med, 2012, 37(3):310–317. doi:10.1097/AAP.0b013e31825735c6.
