1983年,Mazur和Clark首先将胃肠道间质瘤(gastrointestinal stromal tumors,GISTs)区别于其他胃肠道间叶源性肿瘤独立提出。GISTs是消化系统最常见的间叶组织源性肿瘤,独立起源于Cajal细胞(或胃肠道间质干细胞),具有不定向分化特性,在组织形态学上由未分化或多潜能的梭形或上皮样细胞组成。KIT或血小板源性生长因子受体α(PDGFRA)等基因突变,导致酪氨酸激酶持续的活化引起细胞增殖分化失控是其发病的主要原因[1-2]。GISTs发生于消化道的各个部位,病理表现:分界清楚,球形或半球形,质韧,表面呈结节或分叶状,单发或多发,大小不等。GISTs复发率比较高,多为局部复发,周围淋巴结转移较少见。GISTs的基因研究是目前的热点,可以为其诊断及治疗打开新思路。本文对近些年针对GISTs发生、进展、诊断、预后及耐药相关的基因研究进行总结。
1 GISTs发生的相关基因
1.1 KIT
约80%的GISTs中存在KIT基因突变。KIT属III型受体酪氨酸激酶家族,KIT由细胞外结构域,近膜结构域,酪氨酸激酶结构域I和酪氨酸激酶结构域II组成,通过激酶结构域的自身抑制将KIT维持在无活性形式。干细胞因子(SCF)是一种KIT配体,其结合促进ATP与酪氨酸激酶结构域的结合以及近膜结构域中酪氨酸残基的自动磷酸化,SCF-KIT信号通路激活下游途径[3],包括MAP激酶级联和PI3K/Akt途径,前者导致MYC、ELK、CREB和FOS等转录因子的上调,而后者导致细胞周期抑制因子的下调及促进抗细胞凋亡作用[4]。KIT突变常发生于几个区域,包括外显子8、9、11、13、14、15和17,其突变类型以缺失突变、点突变、混合突变和插入突变为主。KIT基因突变以外显子11最为常见,其凭借激酶结构域的自身抑制,保持非活性形式,而外显子11的突变破坏自身抑制并导致KIT的持续激活,与患者年龄、性别、肿瘤原发部位、肿瘤大小、核分裂象及CD34表达有关。KIT基因外显子11突变中,缺失突变主要位于5'端,点突变主要集中在556~560密码子,重复突变主要位于3'端。而KIT基因外显子9突变特点为502~503密码子的重复突变,发生于小肠,瘤体较大,年龄较大(>60岁),多为女性并具有梭形细胞形态。外显子13和17突变均为点突变[5]。现将各突变位点归纳如表1。
表1 KIT突变位点首次发现时间及特点
Table 1 First detection times and significance of mutation sites of KIT gene
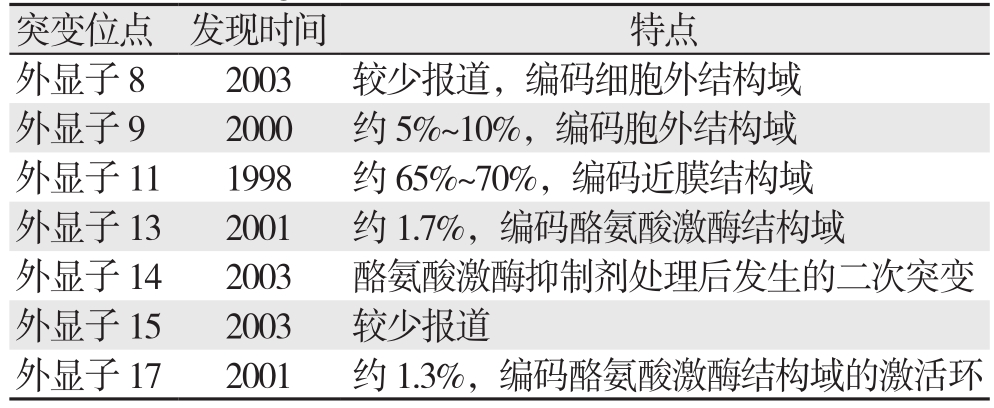
突变位点 发现时间 特点外显子8 2003 较少报道,编码细胞外结构域外显子9 2000 约5%~10%,编码胞外结构域外显子11 1998 约65%~70%,编码近膜结构域外显子13 2001 约1.7%,编码酪氨酸激酶结构域外显子14 2003 酪氨酸激酶抑制剂处理后发生的二次突变外显子15 2003 较少报道外显子17 2001 约1.3%,编码酪氨酸激酶结构域的激活环
1.2 PDGFRA
PDGFRA基因的突变率低于KIT基因,约占总数的5%~10%。PDGFRA基因与KIT基因位于相同染色体的相邻位置,两者氨基酸序列同源性较高,它们的突变以互相排斥的方式发生。PDGFRA突变见于外显子12、14、18突变[6]。现将各突变位点归纳总结如表2。
表2 PDGFRA突变位点首次发现时间及特点
Table 2 First detection times and significance of mutation sites of PDGFRA gene
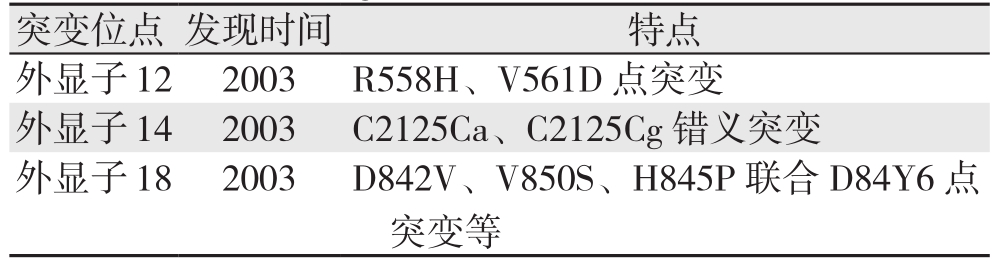
突变位点 发现时间 特点外显子12 2003 R558H、V561D点突变外显子14 2003 C2125Ca、C2125Cg错义突变外显子18 2003 D842V、V850S、H845P联合D84Y6点突变等
PDGFRA突变型GISTs多见于胃,具有上皮样形态,有肥大细胞的浸润,表现为惰性。对于KIT弱免疫反应或不反应的GISTs存在分泌黏液基质的上皮样细胞,即黏液上皮样GISTs[7]。约10%无KIT基因突变的GISTs携带PDGFRA突变,此类GISTs,联合组织形态学及免疫染色法可准确预测PDGFRA突变的基因型[8]。
GISTs中KIT和PDGFRA基因突变率高且突变类型多样,不同外显子突变以及同一外显子的不同突变类型均具有一定的独特性,可为临床个体化治疗提供参考。
1.3 野生型
约10%~15%的GISTs无KIT或PDGFRA基因突变,称为野生型GISTs,CD117阳性或阴性表达,符合GISTs病理学改变[9]。但实际操作中检测技术及标准存在一定的缺陷,所以部分野生型GISTs同样存在KIT和PDGFRA基因突变,有报道[10]野生型GISTs仍可检测到少数KIT外显子8或10的突变。野生型GISTs对酪氨酸激酶抑制剂治疗无效,并发现其中存在琥珀酸盐脱氢酶复合物、BRAF或NF-1等基因突变[11]。
1.3.1 琥珀酸盐脱氢酶(succinate dehydrogenase,SDH)功能缺乏型GISTs 肾癌、副神经节瘤、垂体瘤等存在SDH功能的缺乏,一部分GISTs亦如此。约7.5%的GISTs存在SDH功能缺乏,且其发生不受KIT或PDGFRA基因突变所驱使[12]。约半数野生型GISTs存在SDH功能缺乏。SDH复合体由SDHA,SDHB,SDHC,SDHD 4个亚基组成,在电子传递及能量转化过程中起着重要作用[13]。SDH缺乏型GISTs中,SDH复合体任一亚基的编码基因钝化突变或表观遗传改变(SDHC启动子甲基化)会导致SDH复合体失稳定,使SDH缺乏、琥珀酸盐异常集聚,低氧诱导因子1α(HIF1-α)集聚及HIF1-α受控的原癌基因过度表达,产生假性缺氧信号[14]。同时IGF1R及VEGFR所介导的信号通路上调,抑制IGF1R可诱导细胞凋亡并抑制GISTs细胞中的AKT和MAPK信号传导。该类GISTs主要表现Carney三联征(多发的胃间质瘤、副神经节瘤、肺软骨瘤)或Carney-Stratakis综合征(有遗传倾向的多发的胃间质瘤、副神经节瘤),儿童及青少年多见,仅有10%发生于成人。进展较缓慢,甚至在肿瘤转移数年后,许多患者仍能生存,普遍有淋巴血管侵袭[15-16]。
1.3.2 I型神经纤维瘤病(neurofibromatosis 1,NF-1)相关型GISTs NF-1是17号染色体上NF-1基因突变所致的一类常染色体显性肿瘤综合征,特征为咖啡牛乳色斑,神经纤维瘤,嗜铬细胞瘤以及某些情况下的GISTs。约5%~25%的NF-1患者发生GISTs,而NF-1患者GISTs的患病率是普通人群的至少45倍。NF-1相关型GISTs无KIT/PDGFRA突变,也无SDH功能缺乏 [17]。表现为多中心集中于小肠的肿瘤,核分裂活性较低,预后良好,不易转移。但当此类肿瘤发生于十二指肠时通常表现出侵袭特性,易转移。对于其预后目前仍存在争议。研究发现1/3以上的NFI相关型GISTs表达S-100蛋白,1/5的十二指肠GISTs中S-100呈现不同程度的表达,散发性小肠GISTs中S-100蛋白常不表达(6%)[18]。
1.3.3 BRAF基因突变型GISTs 13%的野生型GISTs中发现BRAF第15号外显子突变,其中BRAF V600E基因突变可见于7%的野生型GISTs中,被认为是一种野生型GIST的发病机制。这种突变常见于甲状腺乳头状癌、黑色素瘤。BRAF是丝氨酸/苏氨酸蛋白激酶RAF家族的一员,涉及RAS-RAF-ERK信号通路,通过激活MPAK通路调控细胞周期和细胞生长信号。BRAF位于KIT下游,V600E位于BRAF激酶区域,突变导致不依赖于KIT的细胞生长,BRAF突变肿瘤对KIT抑制剂治疗并不敏感[19]。突变BRAF与Rac1b,AKT3和其他信号分子协同促进肿瘤细胞活性和增殖。由于BRAF突变在直径4 mm的小GISTs中被发现,它被认为是GIST发展中最早发生的事件之一。研究对比无BRAF基因突变的GISTs,BRAF突变型GISTs并无B-raf蛋白的高表达及MAPK信号通路的优先激活,由于结论的不一致性,BRAF突变是否与野生型GISTs发病相关尚未得到共识。KIT、PDGFRA、BRAF基因均不能被检出突变的GISTs被称之为“三阴型GISTs”。
1.3.4 其他基因突变型GISTs KRAS基因突变可同时存在于KIT突变型GISTs和野生型GISTs中[20]。原发性GISTs中EGFR[21]突变近来被发现,并且不与KIT、PDGFRA、KRAS或BRAF突变重叠。此外,BRCA1和BRCA2是乳腺癌和卵巢癌中的肿瘤抑制基因,有报道[22]BRCA2和GISTs之间有潜在关联。
1.4 家族性GISTs
KIT或PDGFRA的种系突变导致了家族性的GISTs综合征的发生,表现为多发的GISTs、过度色素沉着、肥大细胞和Cajal细胞增生相关性的吞咽困难。在家族性GISTs中发现KIT或突变PDGFRA,KIT突变包括外显子11中的p.V559A,c.1756_1758delGAT和p.W557R,外显子8中密码子419缺失和外显子17中的D820Y取代。PDGFRA突变包括外显子18中的错义突变(D846Y),PDGFRA D846与KIT D820同源,位于酪氨酸激酶结构域内[23]。
2 GISTs疾病进展的相关基因
部分GISTs患者术后5年内复发转移并因此死亡,复发转移是进一步改善GISTs预后的瓶颈[24]。笔者所在团队通过免疫组化检测GISTs中Twist、E钙黏蛋白及N钙黏蛋白的表达并分析其间临床病理特征得知:GISTs发生转移的患者Twist显著高表达而E钙黏蛋白显著低表达,两者的表达与肿瘤的转移密切相关[25]。另一项研究同样支持在远处转移的GISTs组织中E钙黏蛋白显著低表达,而另一转录因子Slug显著高表达,两者呈负相关,与GISTs的转移密切联系[26],高表达slug患者的无复发生存率降低[27]。Wang等[28]通过免疫组化的方法发现KIT外显子11的缺失(包括密码子557-558)与肝脏转移高度相关,密码子557-558过度表达增加CXCR4和ETV1的表达,而CXCR4沉默能够抵抗外显子557-558介导的细胞转移,并且,ETV1沉默可抵抗密码子557-558介导的CXCR4表达。KIT外显子11 557-558密码子依靠增加GISTs细胞中ETV1及CXCR4启动子的结合使CXCR4上调。另外,Brahmi等[29]通过一组随机试验发现KIT(L541)基因型有更高的复发及转移风险。
3 GISTs诊断的相关基因
对于GISTs而言,CD117及DOG1的免疫组化检测可为其提供诊断依据[30],同时CD34、平滑肌肌动蛋白、h-钙黏蛋白、PKCθ也有表达[31],但缺乏特异性。
microRNA(miRNA)参与转录后水平调控,包括转录抑制或促进mRNA降解[32],miRNA表达谱可能成为肿瘤诊断和判断预后的有效生物标志物。miRNA在GISTs中直接参与调控KIT基因的表达水平,并且可阻止GISTs细胞的增殖[33]。在GIST中,miR-16上调及mi-10下调明显[34],相比其他外周组织,GISTs中miR-221以及miR-222也显著下调[35],非野生型GISTs中miR-221以及miR-222的下调更显著。胃与肠道高表达的miRNA不同,KIT及PDGFRA型高表达的miRNA也不相同,miR-210、miR-220c、miR-329、miR-376c高表达于小肠GISTs,miR-15高表达于PDGFRA型GISTs[36]。miRNA的表达模型可用于区分GISTs的分子分类,且与肿瘤风险分级或发生部位密切相关[33],了解GISTs的基因谱对于最合适治疗方案的选择至关重要。
以往,BRAF V600E突变常使用测序相关的技术进行,BRAF V600E突变特异抗体VE1对GISTs中检测BRAF突变具有良好的特异性及敏感性,通过免疫组化即可有效检测BRAF V600E突变[37]。SDH突变型的GISTs可通过免疫组化的方法可检测到SDHB亚基的缺失[17]。
4 GISTs治疗及耐药的相关基因
伊马替尼是治疗不可切除以及转移性GISTs的一线药物,80%的患者对其有临床效应,尤其对于KIT外显子11突变型,而对于KIT外显子9突变型需加大药物剂量[38]。然而,即便多数患者有长久性的临床获益,仍有相当一部分患者肿瘤存在进展。少数患者发生原发耐药和超过半数患者发生继发耐药[39]。
4.1 原发耐药
伊马替尼的原发耐药更多发生于野生型GISTs,原因主要在于野生型GISTs发病机制与KIT或PDGFR基因突变无关[40]。另一原发耐药的GISTs类型为PDGFRA外显子18D842V突变GISTs,这一类型患者对绝大多数酪氨酸激酶抑制剂会发生原发耐药[41],此外,KIT V559D型GISTs 也会发生原发耐药[42]。
4.2 继发耐药
伊马替尼继发耐药是转移性GISTs的治疗难题。目前已明确的继发耐药机制为KIT或PDFRA的继发突变,最常见的突变位点位于ATP结合区(KIT外显子13、外显子14)和kit活化环(外显子17、外显子18),仍有约50% GISTs在伊马替尼耐药之后并未发生KIT基因继发突变[43],但机制尚未明确。抑癌基因PTEN表达下调或丢失可导致AKT过度活化而抵抗肿瘤细胞凋亡,进而对TKIs产生耐药[44]。舒尼替尼对外显子13和14突变KIT型GISTs显示出相对更好的疗效,其对不同突变型GISTs抑制存在差异,可为耐药后GISTs的精准治疗提供药物选择。
5 GISTs预后的相关基因
近10年GISTs患者的预后有较大改善,但部分预后仍较差。其中,KIT基因突变的频率对GISTs的恶性程度有一定影响,其突变频率越高,瘤体越大,核分裂象数也越高[45]。基因改变的确切类型具有很强的临床预后价值。如:KIT外显子9突变比外显子11突变型的GISTs更易发生进展,KIT外显子11缺失型患者5年无病生存率高于KIT外显子11突变型。KIT基因外显子11第557-558密码子的缺失与肿瘤的侵袭性转移表型有关,提示总体预后不良,与其他外显子11突变的患者相比,其5年无复发生存率降低23.8%[46]。KIT基因突变型中,内含子10/外显子11连接缺失将导致p.K550_K558缺失,提示肿瘤可能具有高侵袭性的临床行为。胃部KIT外显子13突变型GISTs的平均体积稍大,危险度相对高。具有PDGFRA突变和KIT外显子11置换或重复型突变的GISTs患者5年复发的风险较低,具有PDGFRA外显子14突变的GISTs预后相对良好(仅限于胃),几乎只有上皮形态[47]。
6 总结与展望
综上,GISTs相关基因分型的研究为其个体化治疗提供了重要依据,基因分型对GISTs的治疗逐渐受到重视,对评估预后具有一定价值。对耐药基因相关的研究,可解决目前伊马替尼等耐药的棘手问题。通过对GISTs基因相关的研究,其发生和进展机制将日渐被阐明,进一步改善GISTs治疗效果,使患者获得长期生存。
[1]Calibasi G, Baskin Y, Alyuruk H, et al.Molecular analysis of the KIT gene in gastrointestinal stromal tumors with novel mutations[J].Appl Immunohistochem Mol Morphol, 2014, 22(1):37–45.doi:10.1097/PAI.0b013e318284a074.
[2]Mavroeidis L, Metaxa-Mariatou V, Papoudou-Bai A, et al.Comprehensive molecular screening by next generation sequencing reveals a distinctive mutational profile of KIT/PDGFRA genes and novel genomic alterations: results from a 20-year cohort of patients with GIST from north-western Greece[J].ESMO Open, 2018,3(3):e000335.doi: 10.1136/esmoopen-2018–000335.
[3]王万川, 廖国庆.基因突变与胃肠道间质瘤研究进展[J].中国普通外科杂志, 2007, 16(9):892–894.doi:10.3969/j.issn.1005–6947.2007.09.018.Wang WC, Liao GQ.Genetic mutation of gastrointestinal stromal tumors[J].Chinese Journal of General Surgery, 2007, 16(9):892–894.doi:10.3969/j.issn.1005–6947.2007.09.018.
[4]Pai T, Bal M, Shetty O, et al.Unraveling the spectrum of KIT mutations in gastrointestinal stromal tumors: AnIndian Tertiary Cancer Center Experience[J].South Asian J Cancer, 2017,6(3):113–117.doi: 10.4103/sajc.sajc_275_16.
[5]Mol CD, Dougan DR, Schneider TR, et al.Structural Basis for the Autoinhibition and STI-571 Inhibition of c-Kit Tyrosine Kinase[J].J Biol Chem, 2004, 279(30):31655–31663.doi: 10.1074/jbc.M403319200.
[6]Indio V, Astolfi A, Tarantino G, et al.Integrated Molecular Characterization of Gastrointestinal Stromal Tumors (GIST)Harboring the Rare D842V Mutation in PDGFRA Gene[J].Int J Mol Sci, 2018, 19(3).pii: E732.doi: 10.3390/ijms19030732.
[7]Tajima S, Ohata A, Koda K, et al.Myxoid epithelioid gastrointestinal stromal tumor harboring an unreported PDGFRA mutation: report of a case and review of the literature[J].Int J ClinExp Pathol, 2015, 8(5):5821–5829.
[8]牛洪欣, 何庆泗.胃肠道间质瘤现代研究进展[J].中国普通外科杂志, 2007, 16(9):895–897.doi:10.3969/j.issn.1005–6947.2007.09.019.Niu HX, He QS.Advances of current research in gastrointestinal stromal tumors[J].Chinese Journal of General Surgery, 2007,16(9):895–897.doi:10.3969/j.issn.1005–6947.2007.09.019.
[9]Steigen SE, Eide TJ, Wasag B, et al.Mutations in gastrointestinal stromal tumors--a population-based study from Northern Norway[J].APMIS, 2007, 115(4):289–298.doi: 10.1111/j.1600–0463.2007.apm_587.x.
[10]Brahmi M, Alberti L, Dufresne A, et al.KIT exon 10 variant (c.1621 A > C) single nucleotide polymorphism as predictor of GIST patient outcome[J].BMC Cancer, 2015, 15:780.doi: 10.1186/s12885–015–1817–5.
[11]Zheng Y, Zheng X, Li S, et al.Identification of key genes and pathways in regulating immune induced diseases of dendritic cells by bioinformatic analysis[J].Mol Med Rep, 2018, 17(6):7585–7594.doi: 10.3892/mmr.2018.8834.
[12]Miettinen M, Lasota J.Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) - a review[J].Int J Biochem Cell Biol, 2014, 53:514–519.doi: 10.1016/j.biocel.2014.05.033.
[13]Pantaleo MA, Astolfi A, Urbini M, et al.Analysis of all subunits,SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST[J].Eur J Hum Genet,2014, 22(1):32–39.doi: 10.1038/ejhg.2013.80.
[14]Hu F, Li H, Liu L, et al.Histone demethylase KDM4D promotes gastrointestinal stromal tumor progression through HIF1β/VEGFA signalling[J].Mol Cancer, 2018, 17(1):107.doi: 10.1186/s12943–018–0861–6.
[15]Belinsky MG, Cai KQ, Zhou Y, et al.Succinate dehydrogenase deficiency in a PDGFRA mutated GIST[J].BMC Cancer, 2017,17(1):512.doi: 10.1186/s12885–017–3499–7.
[16]von Mehren M, Randall RL, Benjamin RS, et al.Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology[J].J Natl ComprCancNetw, 2016, 14(6):758–786.
[17]Garrouche N, Ben Abdallah A, Arifa N, et al.Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review[J].Insights Imaging, 2018, doi: 10.1007/s13244–018–0648–8.[Epub ahead of print]
[18]Sápi Z, Füle T, HajduM, et al.The activated targets of mTOR signaling pathway are characteristic for PDGFRA mutant and wildtype rather than KIT mutant GISTs[J].Diagn Mol Pathol, 2011,20(1):22–33.doi: 10.1097/PDM.0b013e3181eb931b.
[19]Agaimy A, Terracciano LM, DirnhoferS, et al.V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours[J].JClinPathol, 2009, 62(7):613–616.doi: 10.1136/jcp.2009.064550.
[20]Miranda C, Nucifora M, Molinari F, et al.KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors[J].Clin Cancer Res, 2012, 18(6):1769–1776.doi:10.1158/1078–0432.CCR-11–2230.
[21]Qiu HB, Zhuang W, Wu T, et al.Imatinib-induced ophthalmological side-effects in GIST patients are associated with the variations of EGFR, SLC22A1, SLC22A5 and ABCB1[J].Pharmacogenomics J,2018, 18(3):460–466.doi: 10.1038/tpj.2017.40.
[22]Sekido Y, Ohigashi S, Takahashi T, et al.Familial Gastrointestinal Stromal Tumor with Germline KIT Mutations Accompanying Hereditary Breast and Ovarian Cancer Syndrome[J].Anticancer Res, 2017, 37(3):1425-1431.doi: 10.21873/anticanres.11466.
[23]Wali GN, Halliday D, Dua J, etal.Cutaneous hyperpigmentation and familial gastrointestinal stromal tumour associated with KIT mutation[J].Clin Exp Dermatol, 2018, doi: 10.1111/ced.13757.[Epub ahead of print]
[24]Gold JS, Gönen M, Gutiérrez A, et al.Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis[J].Lancet Oncol, 2009,10(11):1045–1052.doi: 10.1016/S1470–2045(09)70242–6.
[25]Ding J, Zhang Z, Pan Y, et al.Expression and significance of twist,E-cadherin, and N-cadherin in gastrointestinal stromal tumors[J].Dig Dis Sci, 2012, 57(9):2318–2324.doi: 10.1007/s10620–012–2186–4.
[26]丁杰, 廖国庆, 张忠民, 等.转录因子Slug、E-钙黏蛋白、N-钙黏蛋白在胃肠道间质瘤中的表达及其意义[J].中华医学杂志, 2012,92(4):264–268.doi:10.3760/cma.j.issn.0376–2491.2012.04.014.Ding J, Liao GQ, Zhang ZM, et al.Expression and significance of Slug, E-cadherin and N-cadherin in gastrointestinal stromal tumors[J].National Medical Journal of China, 2012, 92(4):264–268.doi:10.3760/cma.j.issn.0376–2491.2012.04.014.
[27]Pulkka OP, Nilsson B, Sarlomo-Rikala M, et al.SLUG transcription factor: a pro-survival and prognostic factor in gastrointestinal stromal tumour [J].Br J Cancer, 2017, 116(9):1195–1202.doi:10.1038/bjc.2017.82.
[28]Wang HC, Li TY, Chao YJ, et al.KIT Exon 11 Codons 557–558 Deletion Mutation Promotes Liver Metastasis Through the CXCL12/CXCR4 Axis in Gastrointestinal Stromal Tumors[J].Clin Cancer Res, 2016, 22(14):3477–3487.doi: 10.1158/1078–0432.CCR-15–2748.
[29]Mandrioli M, Mastrangelo L, Masetti M, et al.Characterization of malignant gastrointestinal stromal tumors-a single center experience[J].J Gastrointest Oncol, 2017, 8(6):1037–1045.doi:10.21037/jgo.2017.10.09.
[30]李杰华, 张海添, 陈之白, 等.胃肠道间质瘤KIT、PDGFRA和DOG1基因突变及其对预后的影响[J].中华普通外科杂志, 2017,32(7):569–573.doi:10.3760/cma.j.issn.1007–631X.2017.07.008.Li JH, Zhang HT, Chen ZB, et al.Multiple gene mutation and prognostic factors in gastrointestinal stromal tumors [J].Zhong Hua Pu Tong Wai Ke Za Zhi, 2017, 32(7):569–573.doi:10.3760/cma.j.issn.1007–631X.2017.07.008.
[31]Duensing A, Joseph NE, Medeiros F, et al.Protein Kinase C theta(PKCtheta) expression and constitutive activation in gastrointestinal stromal tumors (GISTs)[J].Cancer Res, 2004, 64:5127–5131.doi:10.1158/0008–5472.CAN-04–0559.
[32]Bartel DP.MicroRNAs: target recognition and regulatory functions[J].Cell, 2009, 136(2):215–233.doi: 10.1016/j.cell.2009.01.002.
[33]Nannini M, Ravegnini G, Angelini S, et al.miRNA profiling in gastrointestinal stromal tumors: implication as diagnostic and prognostic markers[J].Epigenomics, 2015, 7(6):1033–1049.doi:10.2217/epi.15.52.
[34]Subramanian S, Lui WO, Lee CH, et al.MicroRNA expression signature of human sarcomas[J].Oncogene, 2008, 27(14):2015–2026.doi: 10.1038/sj.onc.1210836.
[35]Koelz M, Lense J, WrbaF, et al.Down-regulation of miR-221 and miR-222 correlates with pronounced Kit expression in gastrointestinal stromal tumors[J].Int J Oncol, 2011, 38(2):503–511.doi: 10.3892/ijo.2010.857.
[36]Haller F, von Heydebreck A, Zhang JD, et al.Localization- and mutation-dependent microRNA (miRNA) expression signatures in gastrointestinal stromal tumours (GISTs), with a cluster of co-expressed miRNAs located at 14q32.31[J].J Pathol, 2010,220(1):71–86.doi: 10.1002/path.2610.
[37]时姗姗, 王璇, 夏秋媛, 等.BRAF V600E突变特异性抗体在胃肠道间质瘤中的应用[J].中华病理学杂志, 2016, 45(8):566–570.doi:10.3760/cma.j.issn.0529–5807.2016.08.014.Shi SS, Wang X, Xia QY, et al.Application of BRAF V600E mutation-specific immunohistochemistry in diagnosis of gastrointestinal stromal tumors[J].Chinese Journal of Pathology, 2016, 45(8):566–570.doi:10.3760/cma.j.issn.0529–5807.2016.08.014.
[38]Yan W, Zhang A, Powell MJ.Genetic alteration and mutation profiling of circulating cell-free tumor DNA (cfDNA) for diagnosis and targeted therapy of gastrointestinal stromal tumors[J].Chin J Cancer, 2016, 35(1):68.doi: 10.1186/s40880–016–0131–1.
[39]罗云.胃肠道间质瘤基因突变与分子靶向治疗的研究进展[J].肿瘤预防与治疗, 2015, 28(4):217–222.doi:10.3969/j.issn.1674–0904.2015.04.008.Luo Y.Research advances in gene mutation and molecular targeted therapy of gastrointestinal stromal tumors[J].Journal of Cancer Control and Treatment, 2015, 28(4):217–222.doi:10.3969/j.issn.1674–0904.2015.04.008.
[40]Joensuu H, Wardelmann E, Sihto H, et al.Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial[J].JAMA Oncol, 2017,3(5):602–609.doi: 10.1001/jamaoncol.2016.5751.
[41]Wasielewski K, Wasag B, Wozniak A, et al.Influence of Cytochrome P450, ABC and SLC Gene Polymorphisms on Imatinib Therapy Outcome of Patients with Gastrointestinal Stromal Tumours (GIST)[J].Folia Biol (Praha), 2017, 63(2):78–83.
[42]Zheng S, Huang KE, Pan YL, et al.KIT and BRAF heterogeneous mutations in gastrointestinal stromal tumors after secondary imatinib resistance[J].Gastric Cancer, 2015, 18(4):796–802.doi:10.1007/s10120–014–0414–7.
[43]Kalfusová A, Kodet R.Molecular mechanisms of primary and secondary resistance, molecular-genetic features and characteristics of KIT/PDGFRA non-mutated GISTs[J].Cesk Patol, 2017,53(4):167–173.
[44]Cassinelli G, Zuco V, Gatti L, et al.Targeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cells[J].Curr Med Chem, 2013, 20(15):1923–1945.
[45]Mastalier Manolescu BS, Popp CG, Popescu V, et al.Novel perspectives on gastrointestinal stromal tumors (GISTs)[J].Rom J Morphol Embryol, 2017, 58(2):339–350.
[46]Yan L, Zou L, Zhao W, et al.Clinicopathological significance of c-KIT mutation in gastrointestinal stromal tumors: a systematic review and meta-analysis[J].Sci Rep, 2015, 5:13718.doi: 10.1038/srep13718.
[47]Wozniak A, Rutkowski P, Schöffski P, et al.Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a european multicenter analysis based on ConticaGIST.[J].Clin Cancer Res, 2014, 20(23):6105–6116.doi:10.1158/1078–0432.CCR-14–1677.
