肝内胆管细胞癌(intrahepatic cholangio carcinoma,ICC)是一种临床恶性肿瘤,其发病率约占原发性肝癌的5%~30%。ICC疾病早期临床病症较为隐匿,出现显著特征时大多已属于晚期,失去了最佳治疗时机[1]。因此,以化疗为主的姑息治疗是延长患者生命期、控制病情进展的主要方式。临床常使用吉西他滨与顺铂药物化疗,虽然能够控制病情,但治疗时间较长。文献[2]指出,由于肝脏转移灶的血供来自肝动脉,因此肝动脉灌注(hepatic arterial infusion,HAI)有助于改善患者血清指标,提高远期生存率。本次研究对本院收治的79例ICC患者分别实施5-氟尿嘧啶(5-FU)+顺铂HAI联合吉西他滨静脉化疗或吉西他滨+顺铂进行全身静脉化疗,对比分析两组临床疗效及生存率状况。
1 资料与方法
1.1 一般资料
选取本院收治的79例具有可测量病灶的ICC患者采用随机数字表法分为试验组39例及对照组40例。试验组39例中男22例,女17例;年龄43~75岁,平均年龄(57.6±9.0)岁;高分化6例,中分化9例,未分化24例;单发34例,多发5例;病灶最大径(4.3±1.3)cm。对照组40例中男24例,女16例;年龄41~75岁,平均年龄(59.3±10.5)岁;高分化8例,中分化12例,未分化20例;单发37例,多发3例;病灶最大径(4.1±1.5)cm。两组患者的上述各项资料比较差异均无统计学意义(均P>0.05)。
1.2 纳入与排除标准
纳入标准:⑴ 所有ICC患者入组前均经过B超、CT检查,并结合穿刺活检证实为ICC[3];⑵ 至少具有一个可测量的病灶;⑶ KPS评分≥70分;⑷ 肝功能Child分级均为A、B级;⑸ 研究实施前与患者签订协议书,研究方案获得医学伦理委员会的批准。排除标准:⑴ 发现门静脉癌栓的患者;⑵ 已经发生远处转移的患者;⑶ 患者的肝肾功能不能适应本研究化疗药物;⑷ 预计生存时间<3个月的患者。
1.3 治疗方法
对照组40例患者采用吉西他滨+顺铂进行全身静脉化疗,按照按照25 mg/m2的顺铂(国药准字H20073652,齐鲁制药海南有限公司)给药量溶入500 mL 0.9%氯化钠注射液静脉滴注。将30 mg/m2吉西他滨(国药准字H20093403,江苏豪森有限公司)剂量溶入250 mL 5%葡萄糖注射液,静脉滴注。试验组39例患者采用HAI(5-FU+顺铂)+吉西他滨静脉化疗,用Seldinger技术经患者股动脉穿刺,并插管至肝固有动脉,缓慢灌注化疗药物,方案为5-FU(国药准字H20050511,西安海欣制药有限公司)500 mg/m2+顺铂60 mg/m2联合给药,28 d重复1次。同时给予患者吉西他滨静脉滴注,用法与对照组相一致。两组患者共接受2个疗程治疗,1个月为1个疗程。
1.4 指标及疗效评价
⑴ 检测并比较两组患者化疗治疗前、化疗结束后2周的血清中肿瘤标志物:CEA、CA199、Dickkopf(DKK)的水平变化。⑵ 近期疗效评价标准:参照RECIST实体瘤疗效评价标准分为:完全缓解(CR)、部分缓解(PR)、疾病稳定(SD)、疾病进展(PD);计算缓解率=(CR+PR)/本组样本量×100%;获益率=(CR+PR+SD)/本组样本量×100%。⑶ 不良反应观察标准参考WHO毒副反应5级分级标准:0度,无毒副作用;I度,轻度反应;II度,中度毒副反应;III度,毒副反应重度;IV度,有严重的并发症。分别观察两组患者白细胞、血小板、恶心、呕吐、腹泻、肝功能受损、肝区疼痛、皮疹、口腔黏膜炎的发生率差异。比较2组患者的1、2、3年的生存率差异。
1.5 统计学处理
数据分析在SAS 9.3软件包中处理,正态分布的计量指标采用均数±标准差(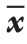 ±s)表示,组间比较采用t检验;计数资料假设检验采用χ2检验;P<0.05为差异有统计学意义。
±s)表示,组间比较采用t检验;计数资料假设检验采用χ2检验;P<0.05为差异有统计学意义。
2 结 果
2.1 两组患者化疗的近期疗效
试验组化疗过程中1例仅完成2周,未能坚持完成,予以退出研究;试验组和对照组均为获得CR患者,试验组15例达到缓解效果、对照组8例缓解;试验组与对照组的缓解率差异无统计学意义(38.46% vs. 20.00%,P>0.05),但试验组的获益率明显高于对照组(79.49% vs. 60.00%,P<0.05)(表1)。
表1 两组患者化疗的近期疗效
Table 1 Short-term results of the two groups of patients
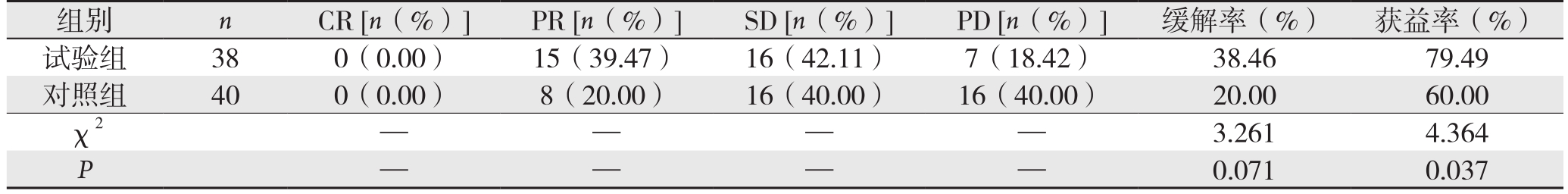
组别 n CR [n(%)] PR [n(%)] SD [n(%)] PD [n(%)] 缓解率(%) 获益率(%)试验组 38 0(0.00) 15(39.47) 16(42.11) 7(18.42) 38.46 79.49对照组 40 0(0.00) 8(20.00) 16(40.00) 16(40.00) 20.00 60.00 χ2 — — — — 3.2614.364 P — —0.0710.037
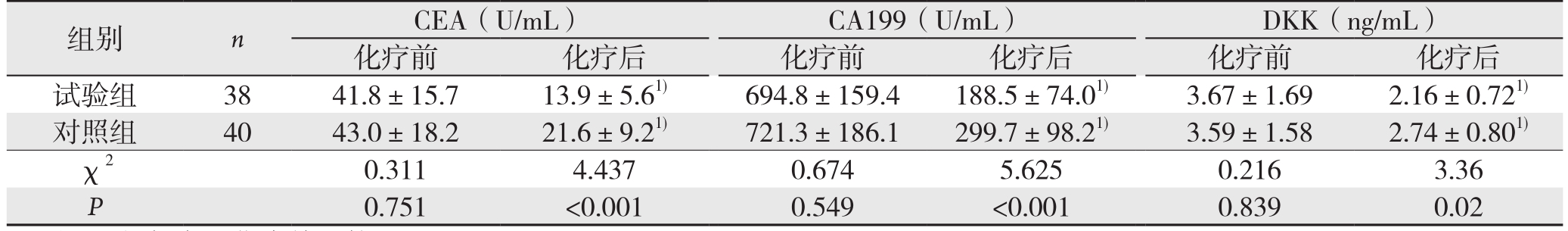
2.2 两组患者化疗前后的CEA、CA199、DKK的水平变化
化疗前,试验组和对照组的CEA、CA199、DKK的水平差异无统计学意义(均P>0.05);两组化疗后CEA、CA199、DKK水平均较化疗前明显降低(均P<0.05),但试验组化疗后的CEA、CA199、DKK的水平均明显的低于对照组(均P<0.05)(表2)。
表2 两组患者化疗前后的CEA、CA199、DKK的水平变化(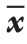 ±s)Table 2 Changes in levels of CEA, CA199 and DKK before and after treatment in the two groups of patients (
±s)Table 2 Changes in levels of CEA, CA199 and DKK before and after treatment in the two groups of patients (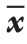 ±s)
±s)
注:1)与本组化疗前比较,P<0.05
Note: 1) P<0.05 vs. pretreatment value of the same group
组别 n CEA(U/mL) CA199(U/mL) DKK(ng/mL)化疗前 化疗后 化疗前 化疗后 化疗前 化疗后试验组 38 41.8±15.7 13.9±5.61) 694.8±159.4 188.5±74.01) 3.67±1.69 2.16±0.721)对照组 40 43.0±18.2 21.6±9.21) 721.3±186.1 299.7±98.21) 3.59±1.58 2.74±0.801)χ2 0.311 4.437 0.674 5.625 0.216 3.36 P 0.751 <0.001 0.549 <0.001 0.839 0.02
2.3 两组患者的远期生存率比较
试验组1、2年生存率分别为63.16%、39.47%,对照组为55.00%、22.50%,差异均无统计学意义(均P>0.05),试验组3年生存率23.68%,对照组为7.50%,试验组3年生存率明显高于对照组(P<0.05)(表3)。
表3 两组患者的远期生存率比较(%)
Table 3 Comparison of the long-term survival rates between the two groups of patients (%)
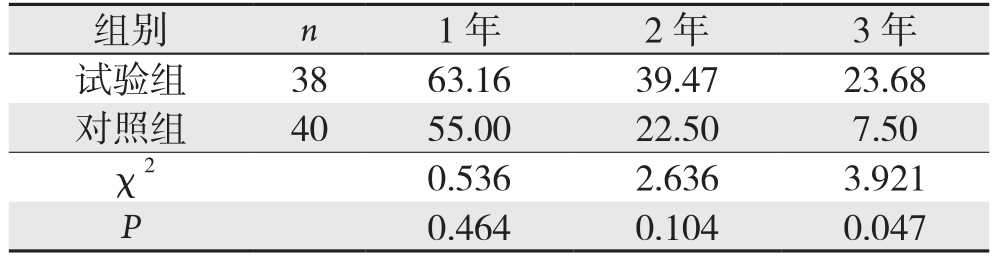
组别 n 1年 2年 3年试验组 38 63.16 39.47 23.68对照组 40 55.00 22.50 7.50 χ2 0.536 2.636 3.921 P 0.464 0.104 0.047
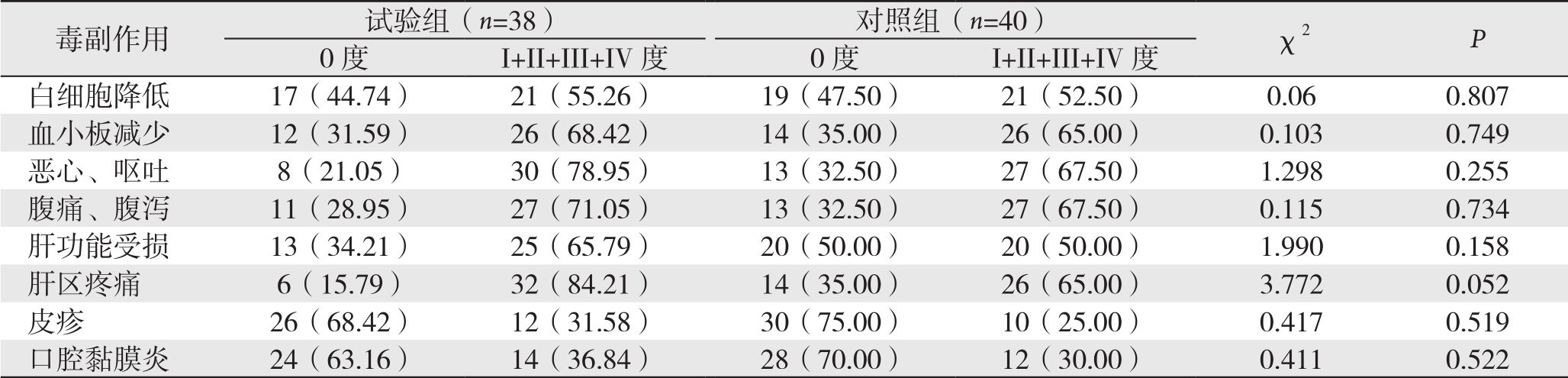
2.4 两组患者的化疗过程中的毒副反应
试验组和对照组患者化疗过程中各项毒副反应的发生率差异均无统计学意义(均P>0.05)(表4)。
表4 两组患者的化疗过程中的毒副反应的发生率[n(%)]
Table 4 Incidence of each toxic and adverse reaction during treatment in the two groups of patients [n (%)]

3 讨 论
ICC也被称为周围型胆管细胞癌,是胆管癌的一种组织学类型,主要是指起源于包含二级胆管在内的末梢侧胆道系统的恶性肿瘤[4]。进展期ICC患者临床病症以肝区肿痛、纳差、乏力、消瘦等为主,对患者的生活质量造成严重影响[5]。文献[6-7]指出,进展期ICC具有恶性程度高、病症隐匿、预后差、中位生存期短等特征,手术是治疗ICC的主要措施,胆优于该疾病与肝外胆管细胞癌一样,早期病症较为隐匿,确诊时已属于晚期,失去了手术治疗的最佳时期。临床常通过化疗治疗ICC患者,延长生存期,提高生存质量[8-9]。全身化疗对进展期ICC的作用尚未明确,系统化疗优于一般性支持治疗,作为姑息治疗手段,全身化疗对延长患者生存时间有限,尚未标准的化疗方案。目前,临床治疗进展期ICC的化疗药物主要为吉西他滨、顺铂、5-氟尿嘧啶(5-FU)[10-11]。
吉西他滨是一种脱氧胞苷类似物,属于新型细胞周期抗代谢药物,是核糖核苷还原酶抑制剂,主要作用于DNA合成期,用于S期,阻止G1期向S期转化,且在细胞内代谢为活性二磷酸盐,竞争性进入DNA双链终止DNA链复制,诱发细胞凋亡[12-13]。顺铂是细胞毒性类药物,具有较强的广谱抗癌作用,通过抑制细胞有丝分裂,抑制癌细胞的DNA复制,损伤其细胞膜结构。但顺铂联合吉西他滨的化疗后患者的生存期尚未获得显著提高,且治疗时间较长[14-15]。
HAI能够直接将5-FU+顺铂药物注入肿瘤供血大动脉内,显著提高肿瘤组织局部的药物浓度,增加杀灭肿瘤细胞数量,同时肝动脉内给药可以减少抗癌药与血浆蛋白结合,缓解局部血流,提高疗效[16-17]。文献[18-19]指出,在动脉灌注时,HAI会增加ICC癌灶局部的化疗药物浓度,约为全身经脉化疗的10~30倍,可杀灭肿瘤细胞。HAI联合吉西他滨具有协同性,能够有效抑制新血管增生,阻止肿瘤增生,消灭播散的亚临床转移灶,缩小局部病灶,降低肿瘤分期[20-21]。本研究中试验组患者的缓解率、获益率均高于对照组患者,且化疗后试验组的CEA、CA199、DKK的水平显著的低于对照组(P<0.05),这说明HAI联合吉西他滨能够有效破坏肿瘤的纤维组织间隔,使得肿瘤组织彻底坏死,抑制迅速发展的癌细胞,改善患者的生存期。本研究发现,试验组患者的1、2年生存率与对照组患者相比均无统计学差异,试验组3年生存率显著的高于对照组,两组患者在化疗过程中出现白细胞降低、血小板减少、肝功能受损、皮疹及口腔黏膜炎等毒副反应的发生率均无统计学意义(P>0.05),这表明进展期肝内胆管细胞癌患者应用HAI结合吉西他滨静脉化疗的效果优于吉西他滨+顺铂进行全身静脉化疗,能够减少肿瘤复发,降低远处转移发生率,增强癌细胞的杀伤敏感性,提高患者机体对毒副作用的耐受性,提高患者生存率。
综上所述,HAI结合吉西他滨全身静脉化疗治疗进展期ICC患者具有显著疗效,能够取得较好的近期疗效,提高远期生存率,不增加毒副反应,值得临床应用。
参考文献
[1] 唐啸. 根治性切除术辅助肝动脉化疗栓塞治疗肝内胆管细胞癌的疗效和预后分析[J]. 临床肝胆病杂志, 2015, 31(2):236–239.doi:10.3969/j.issn.1001–5256.2015.02.022.Tang X. Curative effect and prognostic impact of radical resection assisted by transcatheter arterial chemoembolization in treatment of intrahepatic cholangiocarcinoma[J]. Journal of Clinical Hepatology,2015, 31(2):236–239. doi:10.3969/j.issn.1001–5256.2015.02.022.
[2] 常中飞, 王茂强, 刘凤永, 等. 肝动脉化疗栓塞治疗肝内胆管细胞癌30例临床分析[J]. 中华临床医师杂志:电子版, 2013, (6):2724–2726. doi:10.3877/cma.j.issn.1674–0785.2013.06.082.Chang ZF, Wang MQ, Liu FY, et al. Clinical analysis of hepatic arterial chemoembolization in treatment of intrahepatic cholangiocarcinoma in 30 cases[J]. Chinese Journal of Clinicians:Electronic Edition, 2013, (6):2724–2726. doi:10.3877/cma.j.issn.1674–0785.2013.06.082.
[3] 黄嘉庆, 黄健源. 胆管细胞癌与肝细胞癌血流动力学对比初步研究[J]. 广西医科大学学报, 2013, 30(5):717–719.Huang JQ, Huang JY. Preliminary comparison of hemodynamics between cholangiocarcinoma and hepatocellular carcinoma[J].Journal of Guangxi Medical University, 2013, 30(5):717–719.
[4] Sperling J, Ziemann C, Gittler A, et al. Hepatic arterial infusion of temsirolimus inhibits tumor growth of colorectal rat liver metastases even after a growth stimulating procedure like liver resection[J]. J Surg Res, 2013, 185(2):587–594. doi: 10.1016/j.jss.2013.06.005.
[5] Nouso K, Miyahara K, Uchida D, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan[J]. Br J Cancer, 2013, 109(7):1904–1907.doi: 10.1038/bjc.2013.542.
[6] 颜鹏, 方志勇, 向华. 奥沙利铂及重组人血管内皮抑素经肝动脉灌注联合TACE治疗原发性肝癌[J]. 介入放射学杂志, 2013,22(2):121–124. doi:10.3969/j.issn.1008–794X.2013.02.008.Yan P, Fang ZY, Xiang H. Transhepatic arterial infusion of oxaliplatin and recombinant human endostatin combined with TACE for primary hepatocellular carcinoma[J]. Journal of Interventional Radiology, 2013, 22(2):121–124. doi:10.3969/j.issn.1008–794X.2013.02.008.
[7] 徐力善, 翟博, 方泰石, 等. ALPPS在肝内胆管细胞癌中的应用(附1例报告)[J]. 中国现代普通外科进展, 2015, 18(4):265–272.doi:10.3969/j.issn.1009–9905.2015.04.004.Xu LS, Zhai B, Fang TS, et al. Associating liver partition and portal vein ligation for staged hepatectomy for intrahepatic cholangiocarcinoma: a report of 1 case[J]. Chinese Journal of Current Advances in General Surgery, 2015, 18(4):265–272.doi:10.3969/j.issn.1009–9905.2015.04.004.
[8] 曹震, 谢敏, 冷佳辉. 肝动脉热灌注化疗栓塞治疗原发性肝癌临床观察[J]. 现代肿瘤医学, 2013, 21(2):383–384. doi:10.3969/j.issn.1672–4992.2013.02.53.Cao Z, Xie M, Leng JH. The clinical application of thermochemotherapy combined with TAE in the treatment of primary hepatic carcinoma[J]. Journal of Modern Oncology, 2013,21(2):383–384. doi:10.3969/j.issn.1672–4992.2013.02.53.
[9] 杨广宁, 刘群轶, 翟鹏涛. 肝动脉灌注栓塞术治疗肝癌的临床分析[J]. 中国肿瘤临床与康复, 2014, 21(8):956–958.Yang GN, Liu QY, Zhai PT. Analysis of transcatheter arterial chemoemblization in treatment of hepatocellular carcinoma[J].Chinese Journal of Clinical Oncology and Rehabilitation, 2014,21(8):956–958.
[10] 曾杰宏, 彭思远, 谢博文, 等. 表皮生长因子受体抑制剂联合5-氟尿嘧啶对胆管癌细胞增殖与迁移、侵袭能力的影响[J]. 中国普通外科杂志, 2015, 24(8):1102–1106. doi:10.3978/j.issn.1005–6947.2015.08.009.Zeng JH, Peng SY, Xie BW, et al. Effects of epidermal growth factor receptor inhibitor plus 5-fluorouracil on proliferation,migration and invasiveness of cholangiocarcinoma cells[J]. Chinese Journal of General Surgery, 2015, 24(8):1102–1106. doi:10.3978/j.issn.1005–6947.2015.08.009.
[11] 冯岗, 张羽, 王晋, 等. 肝动脉灌注化疗联合Endostar-碘油栓塞治疗原发性肝癌的研究[J]. 四川医学, 2014, 35(5):566–568.Feng G, Zhang Y, Wang J, et al. Endostar Plus Transcatheter Arterial Chemoembolization (TACE) for Hepatocellular Carcinoma[J].Sichuan Medical Journal, 2014, 35(5):566–568.
[12] 陈耀庭, 姚和瑞, 孙宏亮, 等. 肝动脉灌注化疗联合热疗治疗肝门部胆管癌的临床价值[J]. 中山大学学报:医学科学版, 2014,35(4):539–544.Chen YT, Yao HR, Sun HL, et al. Clinical Evaluation of Hepatic Arterial Infusion Chemotherapy Combined with Hyperthermia for Hilar Cholangiocarcinoma[J]. Journal of Sun Yat-sen University:Medical Sciences, 2014, 35(4):539–544.
[13] 宿濛, 秦宝丽. DC-CIK技术联合奥沙利铂、吉西他滨化疗治疗进展期中央型非小细胞肺癌的临床研究[J]. 现代仪器与医疗,2015, 21(2):31–34. doi:10.11876/mimt201502011.Su M, Qin BL. Clinical analysis of DC-CIK technique combined with chemotherapy of oxaliplatin and gemcitabine for advanced central non-small cell lung cancer[J]. Modern Instruments& Mediccal Treatment, 2015, 21(2):31–34. doi:10.11876/mimt201502011.
[14] 黄大庆, 曾波, 牟忠亮,等. 肝动脉灌注化疗栓塞联合DSA 引导下射频消融术治疗肝癌的疗效[J]. 中国老年学杂志, 2014,34(6):1644–1646. doi:10.3969/j.issn.1005–9202.2014.06.097.Huang DQ, Zeng B, Mou ZL, et al. Clinical efficacy of hepatic arterial infusion combined with DSA-guided radiofrequency ablation in treatment of liver cancer[J]. Chinese Journal of Gerontology, 2014, 34(6):1644–1646. doi:10.3969/j.issn.1005–9202.2014.06.097.
[15] 王飞通, 周兵, 刘小云, 等. 吉西他滨诱导胰腺癌细胞ABCG2表达其化疗耐药的关系研究[J]. 中国普通外科杂志, 2014, 23(3):324–328. doi:10.7659/j.issn.1005–6947.2014.03.012.Wang FT, Zhou B, Liu XY, et al. Relationship between ABCG2 expression induction and chemoresistance of gemcitabine in pancreatic cancer cells[J]. Chinese Journal of General Surgery,2014, 23(3):324–328. doi:10.7659/j.issn.1005–6947.2014.03.012.
[16] 尤振宇, 苏晓辉, 刘洋. DC-CIK生物治疗联合肝动脉灌注化疗治疗肝癌的短期临床观察[J]. 河北医药, 2013, 35(11):1626–1628.doi:10.3969/j.issn.1002–7386.2013.11.010.You ZY, Su XH, Liu Y. Short-term observation of DC-CIK biotherapy combined with hepatic artery infusion chemotherapy in treatment of liver cancer[J]. Hebei Medical Journal, 2013,35(11):1626–1628. doi:10.3969/j.issn.1002–7386.2013.11.010.
[17] 费新平, 鲍鹰. 原发性肝细胞癌术后预防性肝动脉灌注化疗的临床研究[J]. 肝胆胰外科杂志, 2015, 27(2):104–107. doi:10.11952/j.issn.1007–1954.2015.02.005.Fei XP, Bao Y. Clinical research on prophylactic hepatic artery infusion chemotherapy after curative resection in patients with primary hepatocellular carcinoma[J]. Journal of Hepatopancreatobiliary Surgery, 2015, 27(2):104–107.doi:10.11952/j.issn.1007–1954.2015.02.005.
[18] 常中飞, 陈文彰, 孙红梅, 等. 肝动脉灌注化疗治疗丙型肝炎相关性肝癌临床疗效及预后因素分析[J]. 中华临床医师杂志: 电子版, 2015, 9(8):1322–1325. doi:10.3877/cma.j.issn.1674–0785.2015.08.010.Chang ZF, Chen WZ, Sun HM, et al. Prognostic significance of serum gamma-glutamyl transferase before transcatheter arterial chemoembolization in patients with hepatitis C virus related hepatocellular carcinoma[J]. Chinese Journal of Clinicians:Electronic Edition, 2015, 9(8):1322–1325. doi:10.3877/cma.j.issn.1674–0785.2015.08.010.
[19] 刘琪, 武振明, 齐秀恒, 等. 恩度肝动脉灌注联合肝动脉化疗栓塞治疗中晚期原发性肝癌的生存分析[J]. 临床肝胆病杂志, 2015,31(2):225–227. doi:10.3969/j.issn.1001–5256.2015.02.019.Liu Q, Wu ZM, Qi XH, et al. Survival analysis of intrahepatic arterial infusion of Endostar combined with transcatheter arterial chemoembolization for treatment of advanced hepatocellular carcinoma[J]. Journal of Clinical Hepatology, 2015, 31(2):225–227.doi:10.3969/j.issn.1001–5256.2015.02.019.
[20] 崔红利, 杨均, 肖潇, 等. 重组人p53腺病毒经肝动脉灌注化疗治疗中晚期肝癌临床疗效观察[J]. 实用肝脏病杂志, 2015,18(3):237–240. doi:10.3969/j.issn.1672–5069.2015.02.005.Cui HL, Yang J, Xiao X, et al. Clinical evaluation of recombinant adenovirus p53 therapy in combination with transcatheter arterial chemoembolization for patients with primary liver cancer[J].Journal of Practical Hepatology, 2015, 18(3):237–240. doi:10.3969/j.issn.1672–5069.2015.02.005.
[21] 陆小华, 朱小庆, 储玉山,等. 肝癌患者经导管行肝动脉灌注化疗性栓塞治疗前后D-二聚体变化与疗效及预后的关系[J]. 中国肿瘤临床与康复, 2015, 22(9):1025–1028.Lu XH, Zhu XQ, Chu YS, et al. Correlation between D-dimer change before and after intervention treatment with curative effect and prognosis of hepatocellular carcinoma[J]. Chinese Journal of Clinical Oncology and Rehabilitation, 2015, 22(9):1025–1028.
