世界范围内胃癌的发病率在男性和女性中分别位于第3位和第5位[1],在我国胃癌是第二大常见的肿瘤,同时也是仅次于肺癌的第二大病死率的肿瘤[2]。我国人口基数巨大,大多数胃癌患者就诊时已处于进展期,常伴有淋巴结转移和神经侵犯(preineural invasion,PNI)[3]。目前,根治性手术仍然是治疗胃癌的主要手段[4]。PNI是肿瘤常见的转移途径,在胰腺癌、胆管癌、结直肠癌、前列腺癌和头颈部肿瘤中常常可以观察到肿瘤细胞侵犯邻近的神经纤维[5-10]。多项研究证实PNI可作为评价胃癌患者预后的独立因素[11-12],但是影响胃癌PNI的临床病理因素还不清楚。本研究结合文献报道研究胃癌PNI形成的相关临床病理因素,回顾性分析2014年6月—2017年6月在我院就治的509例胃癌患者的年龄、性别、组织分化程度、TNM分期、肿瘤浸润深度、淋巴结转移数目等临床病理资料,探讨胃癌PNI与临床病理因素之间的关系。
1 资料与方法
1.1 一般资料
收集2014年6月—2017年6月在江苏大学附属医院进行外科治疗且临床病理资料完整的胃癌手术患者509例,其中男373例,女136例;年龄28~87岁,平均年龄67岁。患者术前均未发现远处转移,以D2根治术为标准术式,术中探查发现肿瘤侵犯周围脏器或发现网膜转移的,可联合切除以达到根治目的。患者及家属术前均签署手术知情同意书。排除标准:⑴ 术前影像学检查证实肿瘤有远处转移;⑵ 术后发生严重并发症,如吻合口瘘、肺部感染、心脑血管意外等导致死亡患者。
1.2 观察指标及评价标准
患者手术病理组织通过中性福尔马林固定和石蜡包埋,连续3 μm切片,进行苏木精-伊红(HE)染色后由病理科专家进行相关病理诊断。所有患者的分期均统一采AJCC/UICC第7版TNM分期标准。PNI参照文献方法进行评估:肿瘤沿神经纤维生长并包绕≥33%神经直径,或肿瘤细胞侵犯神经纤维的任何一层(包括神经外膜、神经束膜、神经内膜)[9]。将患者的性别、年龄、组织分化程度、浸润深度、淋巴结转移数目和TNM分期等信息录入数据库进行统计学分析。
1.3 统计学处理
采用SPSS 22.0统计软件进行分析。对胃癌患者PNI和临床病理资料进行单因素分析,采用χ2检验判断胃癌PNI和临床病理特征之间的关系。多因素分析采用趋势χ2检验以及二分类Logistic回归模型。P<0.05为差异有统计学意义。
2 结 果
2.1 病理学特征
509例胃癌患者术后病理学检查结果显示:P N I阳性患者2 5 0例,阴性2 5 9例,阳性率为49.1%。经病理确诊的组织分化程度为高分化的56例,中分化组131例,低分化组322例。浸润深度位于黏膜层(T1)的有76例,侵及肌层(T2)的有98例,达浆膜下层的(T3)有68例,侵犯浆膜外的(T4)有267例。无区域淋巴结(N0)转移有185例,1~2个淋巴结转移(N1)有71例,3~6个淋巴结转移(N2)有98例,7个以上淋巴结(N3)转移的有155例。所有患者均采用AJCC/UICC第七版分期标准,结果显示,I期137例、II期99例、III期267期、IV期6例。6例IV期胃癌患者中4例为胃窦部肿瘤侵犯胰腺,2例为胃体肿瘤侵犯大网膜,均行联合脏器或转移灶切除达到根治目的。
2.2 PNI阳性的影响因素
2.2.1 单因素分析结果 比较胃癌PNI阳性和阴性患者的组织分化程度、肿瘤浸润深度、淋巴结转移数目和TNM分期,上述指标比较均有统计学意义(均P<0.001);年龄、性别两者比较,差异无统计学意义(均P>0.05)(表1)。
表1 胃癌PNI相关临床病理因素单变量分析[n(%)]
Table 1 Univariate analysis of clinicopathologic factors for PNI in gastric cancer [n (%)]
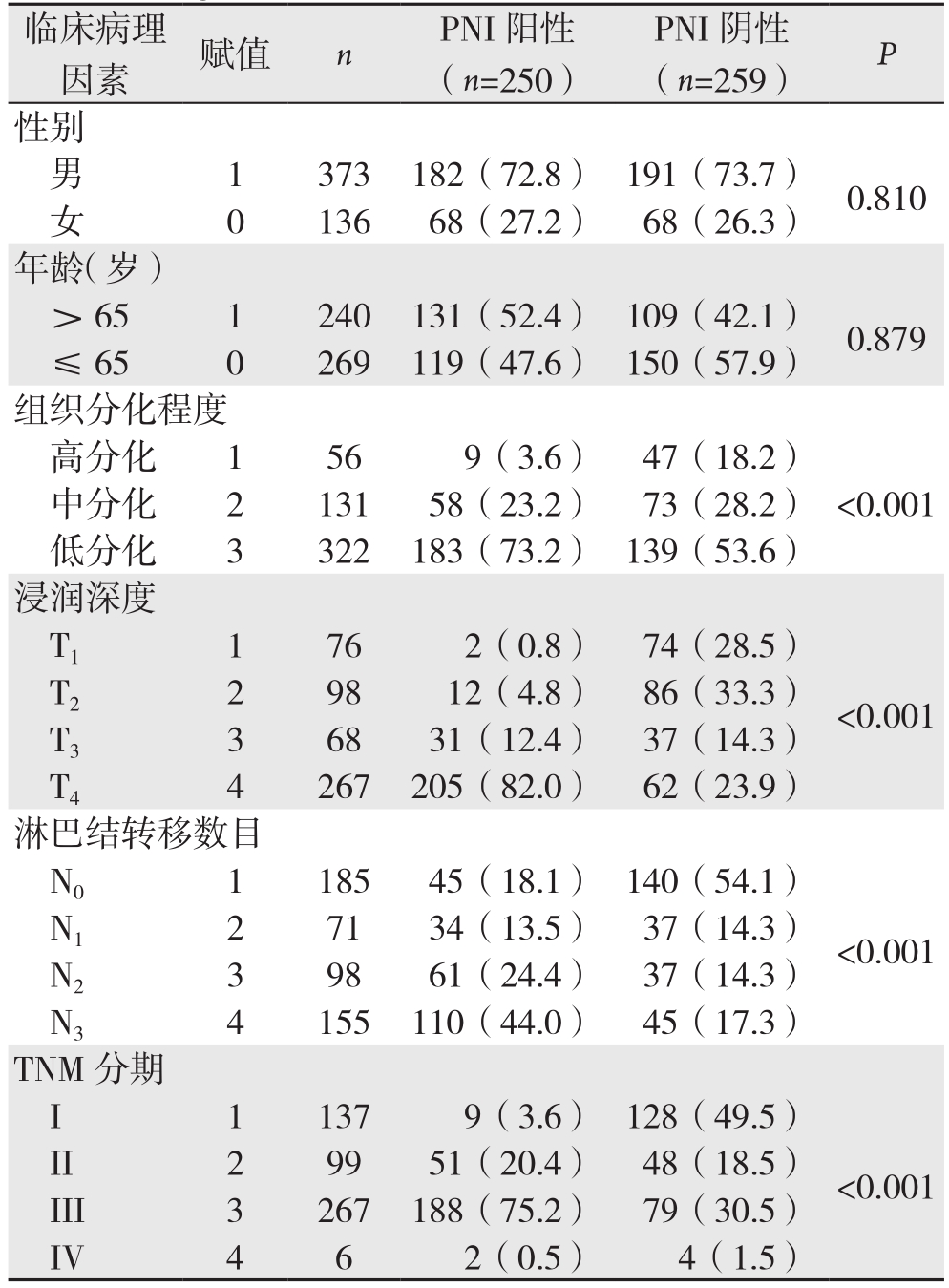
临床病理因素 赋值 n PNI阳性(n=250)PNI阴性(n=259) P中分化 2 131 58(23.2) 73(28.2)低分化 3 322 183(73.2) 139(53.6)浸润深度T1 1 76 2(0.8) 74(28.5)性别男 1 373 182(72.8) 191(73.7) 0.810女 0 136 68(27.2) 68(26.3)年龄(岁)> 65 1 240 131(52.4) 109(42.1) 0.879≤ 65 0 269 119(47.6) 150(57.9)组织分化程度高分化 1 56 9(3.6) 47(18.2)<0.001 T2 2 98 12(4.8) 86(33.3)T3 3 68 31(12.4) 37(14.3)T4 4 267 205(82.0) 62(23.9)淋巴结转移数目N0 1 185 45(18.1) 140(54.1)<0.001 N1 2 71 34(13.5) 37(14.3)N2 3 98 61(24.4) 37(14.3)N3 4 155 110(44.0) 45(17.3)TNM分期I 1 137 9(3.6) 128(49.5)<0.001 II 2 99 51(20.4) 48(18.5)III 3 267 188(75.2) 79(30.5)IV 4 6 2(0.5) 4(1.5)<0.001
2.2.2 趋势χ2检验结果 胃癌组织分化程度、肿瘤浸润深度、淋巴结转移数目和TNM分期与胃癌PNI有统计学意义,且可能存在线性相关(均P<0.001),其中肿瘤浸润深度与胃癌PNI形成的相关性最大(r=0.623,P<0.001)(表2)。
2.2.3 二分类Logistic回归分析 将单因素分析结果中与PNI形成相关的影响因素:组织分化程度、肿瘤浸润深度、淋巴结转移数目和TNM分期等数据代入二分类Logistic回归模型分析,结果显示,肿瘤浸润深度为胃癌PNI发生的独立危险因素(P<0.001)(表3)。
表2 胃癌PNI相关临床病理因素趋势χ2检验[n(%)]
Table 2 Results of χ2 tests for trend of the clinicopothologic factors for PNI in gastric cancer [n (%)]
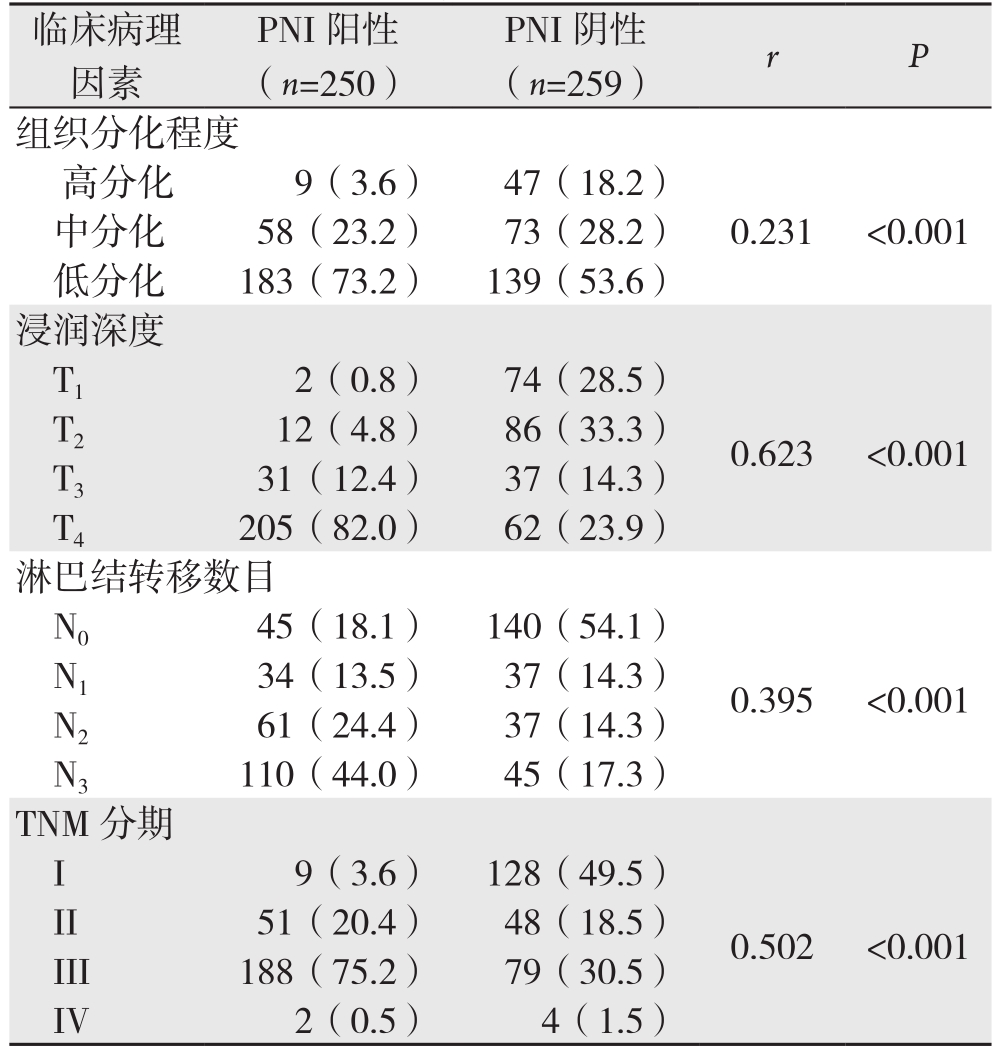
临床病理因素PNI阳性(n=250)PNI阴性(n=259) r P组织分化程度高分化 9(3.6) 47(18.2)中分化 58(23.2) 73(28.2) 0.231 <0.001低分化 183(73.2) 139(53.6)浸润深度T1 2(0.8) 74(28.5)T2 12(4.8) 86(33.3) 0.623 <0.001 T3 31(12.4) 37(14.3)T4 205(82.0) 62(23.9)淋巴结转移数目N0 45(18.1) 140(54.1)N1 34(13.5) 37(14.3) 0.395 <0.001 N2 61(24.4) 37(14.3)N3 110(44.0) 45(17.3)TNM分期I 9(3.6) 128(49.5)0.502 <0.001 II 51(20.4) 48(18.5)III 188(75.2) 79(30.5)IV 2(0.5) 4(1.5)
表3 胃癌PNI相关因素的多变量Logistic回归分析
Table 3 Multivariate Logistic regression analysis of factors
forPNI in gastric cancer
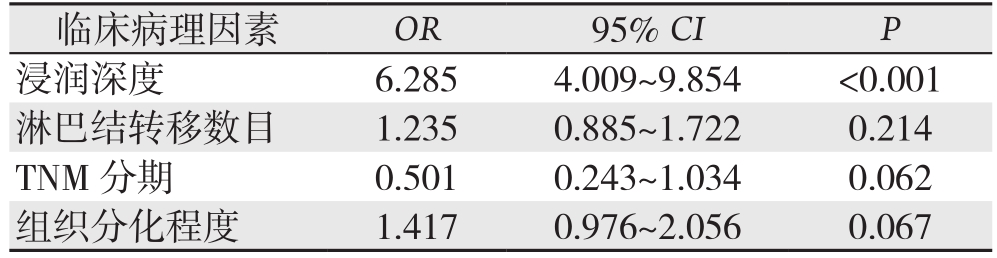
临床病理因素 OR 95% CI P浸润深度 6.285 4.009~9.854 <0.001淋巴结转移数目 1.235 0.885~1.722 0.214 TNM 分期 0.501 0.243~1.034 0.062组织分化程度 1.417 0.976~2.056 0.067
3 讨 论
不同类型肿瘤中PNI的发生率差异较大,其中胰腺癌发生率最高约85%~88%[13]。在总体胃癌手术患者中,术后病理显示PNI的发生率约为50%~60%[14]。本研究结果显示胃癌PNI发生率约为49.1%,这与上述报道基本一致。胃与腹腔神经丛位置临近可能是胃癌PNI发生率较高的原因。PNI目前被认为是肿瘤的一种特殊转移方式,其分子机制目前还未阐明,涉及到肿瘤细胞、基质细胞、周围免疫细胞和神经纤维之间复杂的相互作用[15-17]。PNI的发生发展是一个互动的过程,一方面是神经细胞通过分泌神经生长因子、黏附蛋白等促进肿瘤细胞的增殖与侵袭[6-7, 18-19],另一方面是肿瘤细胞可以导致神经轴突的生长和增粗,从而有利于肿瘤细胞与神经的接触粘附和迁移[20-21]。此外,肿瘤相关巨噬细胞也与PNI的发生发展相关,并且能评估患者的预后[22]。肿瘤伴PNI是患者预后不良的重要因素之一,肿瘤侵犯神经常导致晚期肿瘤患者腰背部剧烈疼痛,生活质量较差[9]。
近年来胃癌的周围神经侵犯越来越受到大家的重视,人们对胃癌PNI形成的临床意义已有较深刻的认识。本研究结果显示随着肿瘤浸润深度加深、分化程度降低、淋巴结转移数目增多和病理学分期增加,胃癌PNI的发生率增高。其它研究报道胃癌PNI也与肿瘤大小、脉管癌栓相关[12]。这提示神经侵犯是胃癌预后不佳的高危因素。经多因素和二分类Logistic回归模型分析后显示,肿瘤浸润深度是影响胃癌PNI独立危险因素。原因可能是胃癌浸润深度越深,与腹腔神经丛接触机率越高。而且进展期胃癌发生PNI的机率明显比早期胃癌高,这证明肿瘤临床分期是胃癌发生PNI的重要影响因素[23]。有研究[24]分析了局部胃癌晚期患者在接受新辅助治疗后再行手术治疗的术后生存时间,发现神经侵犯、淋巴结转移也是影响患者预后的独立因素。
本研究还存在一些不足。既往的研究结果表明:肿瘤的大小和位置,肿瘤细胞类型、脉管癌栓形成和胃癌PNI有显著相关性[25]。本研究中未纳入肿瘤大小、位置、细胞类型等因素,因此需要进行更加全面深入的研究。综上所述,PNI为胃癌侵袭转移的重要途径,PNI阳性与肿瘤浸润深度、组织分化程度、淋巴结转移数目和TNM分期存在线性相关,其中与肿瘤浸润深度相关性最大,但是其具体机制尚不明确,后续有待进一步深入研究其分子机制,以更好地指导肿瘤临床综合治疗,改善患者预后。
参考文献
[1]Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J].CA Cancer J Clin, 2015, 5(2):87-108. doi: 10.3322/caac.21262.
[2]Chen W, Zheng R, Baade PD, et al. Cancer statistics in china,2015[J]. CA Cancer J Clin, 2016, 66(2):115-132. di: 10.3322/caac.21338.
[3]Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in china, 2011[J]. Chin J Cancer Res, 2015, 27(1):2-12. doi:10.3978/j.issn.1000-9604.2015.01.06.
[4]Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2016, 14(10):1286-1312.
[5]Liebl F, Demir IE, Rosenberg R, et al. The severity of neural invasion is associated with shortened survival in colon cancer[J].Clin Cancer Res, 2013, 19(1):50-61. doi: 10.1158/1078-0432.CCR-12-2392.
[6]Abiatari I, DeOliveira T, Kerkadze V, et al. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma[J]. Mol Cancer Ther, 2009, 8(6):1494-1504. doi: 10.1158/1535-7163.MCT-08-0755.
[7]Göhrig A, Detjen KM, Hilfenhaus G, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer[J]. Cancer Res, 2014, 74(5):1529-1540. doi: 10.1158/0008-5472.CAN-13-1012.
[8]He S, He S, Chen CH, et al. The chemokine (ccl2-ccr2) signaling axis mediates perineural invasion[J]. Mol Cancer Res, 2015,13(2):380-390. doi: 10.1158/1541-7786.MCR-14-0303.
[9]Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: A review of the literature[J]. Cancer, 2009, 115(15):3379-3391. doi:10.1002/cncr.24396.
[10]Scanlon CS, Banerjee R, Inglehart RC, et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer[J]. Nat Commun, 2015, 6:6885. doi: 10.1038/ncomms7885.
[11]Selçukbiricik F, Tural D, Büyükünal E, et al. Perineural invasion independent prognostic factors in patients with gastric cancer undergoing curative resection[J]. Asian Pac J Cancer Prev, 2012,13(7):3149-3152.
[12]Bilici A, Seker M, Ustaalioglu BB, et al. Prognostic signif i cance of perineural invasion in patients with gastric cancer who underwent curative resection [J]. Ann Surg Oncol, 2010, 17(8):2037-2044. doi:10.1245/s10434-010-1027-y.
[13]Demir IE, Ceyhan GO, Liebl F, et al. Neural invasion in pancreatic cancer: The past, present and future[J]. Cancers (Basel), 2010,2(3):1513-1527. doi: 10.3390/cancers2031513.
[14]Bapat AA, Hostetter G, Von Hoff DD, et al. Perineural invasion and associated pain in pancreatic cancer[J]. Nat Rev Cancer, 2011,11(10):695-707. doi: 10.1038/nrc3131.
[15]Koide N, Yamada T, Shibata R, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer[J]. Clin Cancer Res, 2006,12(8):2419-2426.
[16]Demir IE, Kujundzic K, Pf i tzinger PL, et al. Early pancreatic cancer lesions suppress pain through cxcl12-mediated chemoattraction of schwann cells[J]. Proc Natl Acad Sci U S A, 2017, 114(1):E85-94.doi: 10.1073/pnas.1606909114.
[17]Zeng L, Guo Y, Liang J, et al. Perineural invasion and tams in pancreatic ductal adenocarcinomas: Review of the original pathology reports using immunohistochemical enhancement and relationships with clinicopathological features[J]. J Cancer, 2014,5(9):754-760. doi: 10.7150/jca.10238.
[18]Xia Q, Bai QR, Dong M, et al. Interaction between gastric carcinoma cells and neural cells promotes perineural invasion by a pathway involving vcam1[J]. Dig Dis Sci, 2015, 60(11):3283-3292.doi: 10.1007/s10620-015-3758-x.
[19]Wang L, Zhi X, Zhu Y, et al. MUC4-promoted neural invasion is mediated by the axon guidance factor Netrin-1 in PDAC[J].Oncotarget, 2015, 6(32):33805-33822. doi: 10.18632/oncotarget.5668.
[20]Ceyhan GO, Demir IE, Altintas B, et al. Neural invasion in pancreatic cancer: A mutual tropism between neurons and cancer cells[J]. Biochem Biophys Res Commun, 2008, 374(3):442-447.doi: 10.1016/j.bbrc.2008.07.035.
[21]Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves[J]. J Natl Cancer Inst,2010, 102(2):107-118. doi: 10.1093/jnci/djp456.
[22]Sugimoto M, Mitsunaga S, Yoshikawa K, et al. Prognostic impact of m2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas[J]. Eur J Cancer, 2014, 50(11):1900-1908. doi: 10.1016/j.ejca.2014.04.010.
[23]De Franco L, Marrelli D, Voglino C, et al. Prognostic value of perineural invasion in resected gastric cancer patients according to lauren histotype[J]. Pathol Oncol Res, 2018, 24(2):393-400. doi:10.1007/s12253-017-0257-8.
[24]Sun Y, Yang L, Wang C, et al. Prognostic factors associated with locally advanced gastric cancer patients treated with neoadjuvant chemotherapy followed by surgical resection[J]. Oncotarget, 2017,8(43):75186-75194. doi: 10.18632/oncotarget.20660.
[25]Jiang N, Deng JY, Liu Y, et al. Incorporation of perineural invasion of gastric carcinoma into the 7th edition tumor-node-metastasis staging system[J]. Tumour Biol, 2014, 35(9):9429-9436. doi:10.1007/s13277-014-2258-5.
