胆管癌是指源于胆道系统的恶性肿瘤,过去10年间发病率增加近20%,根据其发生部位可分为肝内胆管癌(intrahepatic cholangiocarcinoma,ICC)、肝门部胆管癌和远端胆管癌[1-7]。ICC是指源自肝内胆管上皮细胞的恶性肿瘤,约占胆管癌的10%。ICC发病风险因素主要有肝胆管结石、原发性硬化性胆管炎、肝炎病毒感染(乙型与丙型)、肝硬化、肥胖和饮酒等[8]。ICC临床症状因发生部位不同而异;末梢型胆管癌早期多无临床表现,晚期可有上腹部隐痛、肝大、体质量下降等症状。ICC恶性程度高,具有极强的侵袭和淋巴结转移特性。ICC早期诊断困难,许多患者确诊时已属中晚期。手术治疗是目前治愈胆管癌唯一可能的方法,但ICC根治性手术后5年生存率仅有9.5%[9]。随着肿瘤分子学的发展,已有诸多肿瘤可通过分子标记物检测达到早期诊断和指导治疗的目的。本文综述ICC相关分子标记物的研究现状和最新进展。
1 ICC 遗传学分子研究多聚焦于基因的变化
肿瘤分子遗传学是研究生物大分子及其产物与癌的关系,包括癌基因、抑癌基因及其产物,肿瘤微环境与癌症的关系;原癌基因在人体内呈低表达状态,并发挥重要的生理功能;原癌基因被激活后大量表达,发生序列反应,导致细胞癌变。抑癌基因具有拮抗原癌基因作用,维持正负调节的相对稳定,抑癌基因失去作用时可发生癌变。近几年来,发现了一些ICC相关原癌基因、抑癌基因以及肿瘤微环境中的细胞因子。
1.1 抑癌基因
1.1.1 p53 基因 定位于人类17 号染色体,含有11 个外显子。p53 调控细胞周期G1 和G2/M。p53基因发生突变时,细胞生长将会失去调控,从而诱导肿瘤形成。P53 蛋白可引起细胞周期停滞并上调Bax 表达水平以及下调Bcl-2 表达,从而抑制肿瘤生长。p53 常失去杂合性及发生钝化突变导致胆管上皮细胞癌变[10]。王新根等 [11]通过收集胆管癌52 例,其中ICC48 例,肝外胆管癌(ECC)4 例,选取非肿瘤性疾病胆囊及肝内胆管增生病例26 例作为对照组研究发现,胆管癌及对照组p53 表达率分别为89%、54%,然而在中等以上表达强度的统计中则胆管癌组表达率约60%,对照组则低至8%。
1.1.2 p16 基因 是存在于染色体9p21 上的抑癌基因,P16 蛋白的作用是能与cyclin D1 竞争性结合 CDK4,阻止细胞进入G1/S 期,从而抑制细胞过度增殖。当p16 基因发生变异时,细胞将失去这种抑制作用而出现无限增殖和恶性转化。Ishikawa等[12] 根据胆管上皮内瘤变的程度分组后发现,随着上皮内瘤变级别的升高,p16 的表达率逐渐降低,p16 的缺失将加快肿瘤的形成。谷化平等[13]报道,胆管癌中P16 蛋白阳性表达率为42.0%,明显低于正常胆管上皮的90.0%(P<0.05)。有文献[14]报道,p16 的失表达现象在ICC 比ECC 中更常见。
1.1.3 BRCA1相关蛋白1(BRCA1-associated protein 1,BAP1)基因BAP1基因定位于人染色体3p21,其编码的产物BAP1 由729 个氨基酸组成的去泛素化酶, 可作用于H2A、HCF、INO80、KLF5、MCRS1、r-tubulin 以及BAP1 自身,如BAP1 可去泛素化宿主细胞因子1 进而解除宿主细胞因子1 对E2F 反应性启动子活性的抑制作用,从而促进细胞周期进展和细胞增殖[15]。BPA1 是肾癌、眼部肿瘤、胆管癌、间皮瘤等肿瘤的抑癌基因。最近相关专家提出BAP1 突变倾向于肝内胆管细胞癌。Andric 等[16]对211 例ICC 患者进行了BAP1 免疫组织化学检查,研究显示26% 的ICC中BAP1 存在突变。这结果与Jiao 等[17] 报告的失活突变的发生率非常相似。最近一次研究[18] 也发现BAP1 的低表达促进了ICC 细胞系中的细胞增殖,迁移和侵袭能力。
1.1.4 DPC4/SMAD4 DPC4 基因,又称SMAD4基因位于染色体18q21.1 上,其编码的SMAD4 蛋白的主要作用是参与了转化生长因子β(TGF-β)超家族的信号在细胞内的传导。TGF-β 可调节细胞的增殖、分化和凋亡,在组织的发育、再生修复中起重要作用。DPC4/SMAD4 基因的表达缺失使得TGF-β 通路基因转录失活,造成肿瘤形成。另外DPC4 还有下调血管内皮生长因子的表达,从而抑制肿瘤血管的生成的作用。DPC4/SMAD4 基因的突变在食管癌[19]、胃肠道神经内分泌肿瘤[20]、胰腺导管腺癌[21] 都有报道。然而在ICC 方面研究较少,Lee 等[22] 采用免疫组化和PCR 技术检测正常胆道细胞和胆管癌的DPC4/SMAD4 表达,发现胆管癌的表达缺失率明显高于正常胆管细胞。但并不影响DPC4/SMAD4 突变靶点作为治疗ICC 的一个新的思路。
1.2 原癌基因
1.2.1 Ras 致癌基因家族 包括H-ras、K-ras 和N-ras,其功能是可编码具有GTP 酶活性的P21 蛋白。当Ras 基因突变时,P21 蛋白的GTP 酶活性减弱,对细胞的增殖、分化、迁移和凋亡等起到重要调节作用。胆管癌相关的Ras 基因主要是K-ras基因。Chen 等[23] 对86 例ICC 手术患者资料进行回顾性分析发现,K-ras 基因突变是ICC 患者预后的重要指标。伴有该基因突变的手术患者,术后中位生存时间为5.9 个月,而不伴该基因突变的患者生存时间为19.0 个月。
1.2.2 c-erbB-2又称为neu基因或HER-2 基因:定位在17q21 染色体上,其编码的P185 蛋白具有酪氨酸激酶活性 , 在上皮细胞生长信号中起调剂作用[ 24]。文献[25] 报道,c-erbB-2 过度表达可以激活信使系统,促使细胞增殖失控, 其过表达与多种肿瘤的预后相关。郑军等[26] 研究发现原癌基因c-erbB-2 在21 例原代培养人胆管癌细胞中有 18 例(86%)表达阳性,3 例表达阴性,6 例原代培养人正常胆管细胞中c-erbB-2 蛋白的表达均为阴性。表明c-erbB-2 在胆管癌中高表达。
1.2.3 C-ROS癌基因1(c-ROS-oncogene 1,ROS1) 定位于人体第6 号染色第q22 区,编码酪氨酸蛋白激酶。当ROS1 基因编码异常时,可致持续激活ROS1 酪氨酸激酶区及下游PI3K/AKT/mTOR、JAK 等信号通路,从而促进肿瘤的发生与发展。有研究[27] 发现约有9% 的胆管癌组织中发现ROS1 基因突变。朱垒等[28] 对ICC 细胞株中ROS 蛋白的表达情况发现,ROS 融合基因阳性表达率较低。但在ICC 中,ROS 融合蛋白仍是一种强有力的肿瘤蛋白。越来越多的研究也表明ROS融合基因一个有效治疗ICC 靶位点。
1.2.4 异柠檬酸脱氢酶(isocitrate dehydrogenase,IDH)1和2(IDH1/IDH2) IDH1/IDH2 是催化异柠檬酸氧化脱羧成α-酮戊二酸的酶(α-ketoglutarate,α-KG)、还原性烟酰胺腺嘌呤二核苷酸磷酸。IDH1/2 突变导致细胞内由α-KG 生成的2-羟基戊二酸(hydroxyglutarate,2-HG)。由于2-HG 可竞争性抑制α-KG 依赖性酶使得DNA 和组蛋白的异常高甲基化。IDH1/2基因突变发生在各种类型的恶性肿瘤包括神经胶质瘤[29],软骨肉瘤[30],ICC[31] 和急性骨髓性白血病[32]。IDH 突变导致2-HG 水平上调,促使HIF1α 活性增强,从而使得DNA 及组蛋白处于高甲基化状态,可能是导致胆管癌发生发展的原 因[33]。以往认为IDH1/IDH2 突变在实体器官肿瘤中很少见[34]。然而,最近几次突变图谱的研究[35-37]表明,IDH 突变是胆管癌较常见的基因畸变。且ICC 相对ECC 常见。
综上,目前ICC发现的突变肿瘤抑制基因主要有ARID1A、ARID1B、BAP1、PBRM1、TP53、STK11和PTEN,以及致癌基因,即IDH1、IDH2、KRAS、BRAF和PIK3CA[38]。根据ICC的DNA基因突变情况,因而针对ICC的靶向治疗有多种可选择的靶点,而目前有大量相关基础研究和临床试验正在进行中,其中部分研究已显示了较好的苗头[39]。
2 miRNA 在ICC 中的研究进展
在2001年人类基因组测序完成后,研究人员发现蛋白质编码序列仅占人类基因组的2%,其剩余98%的序列为非编码序列,仅能转录成RNA而不被翻译为蛋白质[40]。随着近年来高通量转录组学研究的进展,发现了大量的miRNA。miRNA为DNA在转录过程中,能转录但不被翻译成的蛋白质长度约20~24个核苷酸的非编码RNA。miRNA通过与目标mRNA碱基完全或近乎完全的互补可诱导靶基因mRNA的降解,而部分互补则通过阻断核糖体与mRNA结合而抑制mRNA的翻译,从而阻止相关基因的表达[41]。miRNA在细胞生长、组织分化等方面起重要的调控作用。
miRNA在ICC的发病中具有重要作用,如miR-31[42]、miRNA-320[43]、miR-29[44]等在肿瘤的增殖和抗凋亡中起重要作用。Mott等[45]发现miR-29在胆管癌中呈低表达,其抑癌机制为m i R-2 9 抑制Mcl-1促进肿瘤坏死因子引起的凋亡;miR-29 表达下调可能与c-Myc、Hedgehog及NF-к B等通路的开放相关[44]。多种miRNA与肿瘤的侵袭和转移密切相关,如miR-21[46-48]、miR-124[49]、 miR-214[50]、miR376c[51]等在ICC与肿瘤的转移有些密切的关联。肝吸虫相关性胆管癌中发现miR-21通过PDCD4、TIMP3具有促进增殖作用[52]。Liu等[53]对胆管癌RBE及QBC939两种细胞株进行miRNA-122对照实验,证实miRNA-122过表达对两种细胞株具有促进侵袭、转移能力的作用。Li等[54]发现miR-221作用于PTEN形成β-catenin/c-Jun闭环通路,正反馈促进胆管癌的侵袭转移。由于miRNA表达与肿瘤发生、发展有密切的关联,因此,以这些异常的miRNA为靶点可能是治疗ICC有效的策略。miRNA相关片段在ICC中的作用见表1。
表1 miRNA 相关片段在ICC 中的作用
Table 1 Actions of the relevant miRNAs in ICC
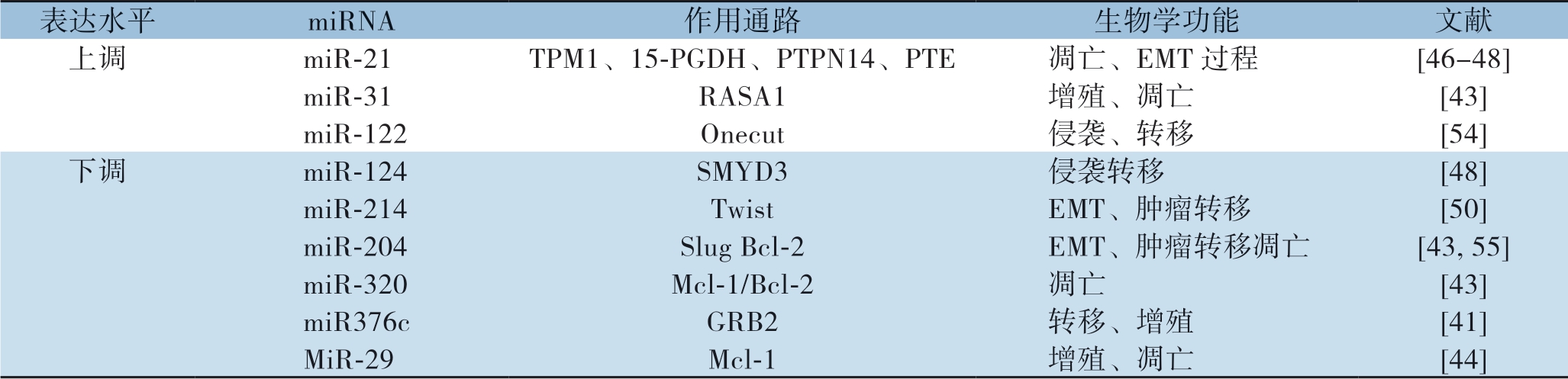
表达水平 miRNA 作用通路 生物学功能 文献上调 miR-21 TPM1、15-PGDH、PTPN14、PTE 凋亡、EMT 过程 [46-48]miR-31 RASA1 增殖、凋亡 [43]miR-122 Onecut 侵袭、转移 [54]下调 miR-124 SMYD3 侵袭转移 [48]miR-214 Twist EMT、肿瘤转移 [50]miR-204 Slug Bcl-2 EMT、肿瘤转移凋亡 [43, 55]miR-320 Mcl-1/Bcl-2 凋亡 [43]miR376c GRB2 转移、增殖 [41]MiR-29 Mcl-1 增殖、凋亡 [44]
3 蛋白修饰
蛋白修饰多在蛋白-N末端,如乙酰化、甲基化、磷酸化等共价修饰,这些修饰使得基因表达产物发生改变;这些蛋白修饰与DNA抑制与失活相关。蛋白脱乙酰基酶(HDAC)是组织蛋白修饰中比较重要的部分;多种恶性肿瘤中HDAC均有过表达的现象,如乳腺癌中HDAC-1,结肠癌中HDAC-2/3均存在过量表达,胆管癌中HDAC也具高表达状态,HDAC-1的过度表达与ICC恶性程度、和预后差相关[56]。朱鹏等[57]运用HDAC抑制剂曲古菌素A(TSA)作用于胆管癌细胞系QBC939发现HDACI(TSA)通过抑制肿瘤细胞内组蛋白去乙酰化酶,使细胞内组蛋白过乙酰化,诱导肿瘤细胞的凋亡。已有超过80种HDACI应用于临床,可单用或者联合其他化疗一起使用,比较常用的HDAC药物有伏立诺他、缩酚酞、MS-275[58]等。然而,在ICC中有关HDAC研究比较少。组蛋白修饰可能引起广泛的基因表达谱差异并导致ICC易转移以及出现广泛的化疗耐药,其机制仍值得进一步探索[52]。
4 ICC 的分子靶向治疗
外科手术是当前可能治愈ICC的唯一的方法,然而术后总体预后差。2017年版NCCN胆道系统恶性肿瘤指南指出:⑴ 对于可切除ICC,手术切除和淋巴结清扫是唯一可能的治愈手段。⑵ 对于不可切除者可予以氟嘧啶或吉西他滨为基础的化疗、局部区域疗法(射频消融术、经动脉化疗栓塞、药物洗脱微球动脉化疗栓塞和钇-90微球的肝动脉放疗性栓塞、肝动脉输注化疗等综合治疗。⑶ 对于不可切除者,联合吉西他滨+西妥昔单抗可获得较好的疗效[59]。对于ICC术后患者,目前仍没有明确的最佳辅助治疗策略与标准方案。
随着对胆管癌分子机制的深入理解,人们开始关注ICC的分子靶向治疗。生物靶向治疗是针对特定的致癌位点(基因片段、抗原/受体或肿瘤微环境),通过设计相关的治疗药物进入特定的致癌位点,使肿瘤细胞特异性死亡,而不伤及正常组织或细胞的干预治疗策略。临床常用的靶向药物主要以EGFR、VEGFR、PDE-FR、BRAF、MEK、mTOR、LI-6、COX-2等为作用靶点[60]。肿瘤基因靶向治疗,主要有IDH1/IDH2抑制剂。选择性IDH1抑制剂AGI-5198阻断了这种酶产生代谢产物2-HG的能力,导致IDH1突变细胞的生长受损[61]。同样,选择性突变IDH2抑制剂AGI-6780在造血细胞系中诱导分化[62]。IDH1突变的受试者进行AG-120的晚期多中心,随机,双盲,安慰剂对照研究(NCT02989857),AG-221再IDH2突变的ICC实验正在进行中(NCT02273739)。肿瘤生物免疫靶向治疗,主要包括:⑴ 以被动免疫为主的表皮生长因子受体(epidermal growth factor receptor,EGFR)和血管内皮生长因子(vascular endothelial growth factor,VEGF)的单克隆抗体[63]。EGFR抗体(西妥昔单抗)和/或抗VEGF抗体(贝伐单抗)联合临床一线化疗药物取得了一定的疗效;⑵ 以主动免疫为主的激活的T淋巴细胞、树突状细胞疫苗、黏蛋白(mucin1,MUC1)的树突状细胞疫苗或胆管癌相关抗原Wilms肿瘤基因(Wilms' tumor gene,WT1)都取得了可喜的疗效,患者副作用低,总体生存率提高[64]。针对肿瘤微环境炎症因子的靶向治疗,ICC的病理基础是胆管内皮细胞长期受到慢性炎症刺激,而其中扮演重要角色的炎症因子是IL-6、COX-2[65]。塞来考昔是一种选择性COX-2抑制剂,李勤裕等[66]研究了塞来考昔联合EGFR酪氨酸激酶抑制剂埃罗替尼对胆管癌细胞株QBC939的作用,发现两药联合可使癌细胞内EGFR的下游分子p-MAPK下调,Ki-67、VEGFR的表达的降低,两者具有协同作用,可显著抑制肿瘤细胞生长,抑制肿瘤新生血管形成。由于ICC癌的发生、发展涉及多个基因的突变或者多个信号通路的相互作用,目前对胆管癌的生物治疗大多仍处于实验研究阶段,临床应用较少。ICC存在基因变异的个体差异,进展期胆管癌应根据基因检测结果实施个体化分子靶向治疗[56]。
5 总结与展望
ICC形成、生长、侵袭和转移等过程是一个多因素刺激、多个关键因子参与的过程。ICC手术治疗和化疗效果均不理想。尽管现阶段肿瘤细胞分子生物学研究越来越深入,但是ICC发病的分子生物学发病机制并不明确。表观遗传学研究提示基因遗传变异、miRNA和组蛋白修饰的改变与ICC的发病机制有关,寻求ICC靶向治疗可能成为治疗ICC新的思路。未来新技术、新方法的运用,找到引发ICC发生和发展中的关键调节子并将其作为治疗ICC的有效靶分子,或找出诊断ICC发生的关键因子一定为取得重大突破。将深刻改变未来ICC的治疗模式。
[1] Razumilava N, Gores GJ. Cholangiocarcinoma[J]. Lancet, 2014, 383(9935):2168-2179. doi: 10.1016/S0140-6736(13)61903-0.
[2] Tang H, Lu W, Li B, et al. Prognostic significance of neutrophiltolymphocyte ratio in biliary tract cancers :a systematic review and meta-analysis[J]. Oncotarget, 2017, 8(22):36857-36868. doi: 10.18632/oncotarget.16143.
[3] Li B, Tang H, Zhang A, et al. Prognostic Role of Mucin Antigen MUC4 for Cholangiocar-cinoma : A Meta-Analysis[J]. PLoS ONE, 2016, 11(6):e0157878. doi: 10.1371/journal.pone.0157878.
[4] Cai Y, Cheng N, Ye H, et al. The current management of cholangiocarcinoma:A comparison of current guidelines[J]. Biosci Trends, 2016, 10(2):92-102. doi: 10.5582/bst.2016.01048.
[5] Wang H, Liu W, Tian M, et al. Coagulopathy associated with poor prognosis in intrahepatic cholangiocarcinoma patients after curative resdanguanection[J]. Biosci Trends, 2017, 11(4):469-474. doi: 10.5582/bst.2017.01080.
[6] 李会星. 肝门部胆管癌的诊治进展[J]. 解放军医学院学报, 2014, 35(1):98-101. doi:10.3969/j.issn.2095-5227.2014.01.031. Li HX. Advances in diagnosis and treatment of hilar cholangiocarcinoma[J]. Academic Journal of Chinese PLA Medical School, 2014, 35(1):98-101. doi:10.3969/j.issn.2095-5227.2014.01.031.
[7] Global Burden of Disease Cancer, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study[J]. JAMA Oncol, 2017, 3(4):524-548. doi: 10.1001/jamaoncol.2016.5688.
[8] 陈世成, 符国珍, 周帅. 胆管癌危险因素研究进展[J]. 海南医学, 2015, 26(9):1334-1338. doi:10.3969/j.issn.1003-6350. 2015.09.0478. Chen SC, Fu GZ, Zhou S. Research progress of risk factors for cholangiocarcinoma[J]. Hainan Medical Journal, 2015, 26(9):1334-1338. doi:10.3969/j.issn.1003-6350.2015.09.0478.
[9] 刘晶. 肝门部胆管癌外科诊治及预后分析[D]. 银川: 宁夏医科大学, 2013. Liu J. Diagnosis, treatment and prognosis analysis of hilar cholangiocarcinoma[D]. Yinchuan: Ningxia Medical University, 2013.
[10] 史保宾, 吴阳, 唐哲, 等. 胆管癌发病机制的研究进展[J]. 癌症进展, 2015, 13(1):39-43. doi:10.11877/j.issn.1672-1535.2015. 13.01.08. Shi BB, Wu Y, Tang Z, et al. Research progress of pathogenesis for cholangiocarcinoma[J]. Oncology Progress, 2015, 13(1):39-43. doi:10.11877/j.issn.1672-1535.2015.13.01.08.
[11] 王新根, 周有俭, 徐胜美, 等. P53和CEA双染色在胆管癌病理诊断中的应用[J]. 中国热带医学, 2015, 15(11):1383-1385. doi:10.13604/j.cnki.46-1064/r.2015.11.26. Wang XG, Zhou YJ, Xu SM, et al. The application of P53 and CEA double staining in the pathological diagnosis of cholangiocarcinoma[J]. China Tropical Medicine, 2015, 15(11):1383-1385. doi:10.13604/j.cnki.46-1064/r.2015.11.26.
[12] Ishikawa A, Sasaki M, Sato Y, et a1. Frequent pl6ink4a inactivationsan early and frequent event of intraductal papillary neoplasm of the liver arising in hepatolithiasis [J]. Hum Pathol, 2004, 35(12):l505-1514.
[13] 谷化平, 黄勇, 尚培中, 等. 细胞周期素D1和p16蛋白表达与胆管癌转移和预后的关系[J]. 肝胆胰外科杂志, 2013, 25(3):219-221. doi:10.3969/j.issn.1007-1954.2013.03.013. Gu HP, Huang Y, Shang PZ, et al. Re lationship between the expression of Cyclin D1 and p16 protein in human cholangiocarcinomas and its metastasis and prognosis[J]. Journal of Hepatopancreatobiliary Surgery, 2013, 25(3):219-221. doi:10.3969/j.issn.1007-1954.2013.03.013.
[14] Karamitopoulou E, Tornillo L, Zlobec I, et al. Clinical significance of cell cycle- and apoptosis-related markers in biliary tract cancer: a tissue microarray-based approach revealing a distinctive immunophenotype for intrahepatic and extrahepatic cholangiocarcinomas [J]. Am J Clin Pathol, 2008, 130(5):780-786. doi: 10.1309/AJCP35FDCAVANWMM.
[15] Qin J, Zhou Z, Chen W, et al. BAP1 promotes breast cancer cell proliferation and metastasis by deubiquitinating KLF5[J]. Nat Commun, 2015, 6:8471. doi: 10.1038/ncomms9471.
[16] Andrici J, Goeppert B, Sioson L,et al. Loss of BAP1 Expression Occurs Frequently in Intrahepatic Cholangiocarcinoma[J]. Medicine (Baltimore), 2016, 95(2):e2491. doi: 10.1097/MD.0000000000002491.
[17] Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1,ARID1A and PBRM1 in intrahepatic cholangiocarcinomas[J]. Nat Genet, 2013, 45(12):1470-1473. doi: 10.1038/ng.2813.
[18] Chen XX, Yin Y, Cheng JW, et al. BAP1 acts as a tumor suppressor in intrahepatic cholangiocarcinoma by modulating the ERK1/2 and JNK/c-Jun pathways[J]. Cell Death Dis, 2018, 9(10):1036. doi:10.1038/s41419-018-1087-7.
[19] 宋文庆, 俞岚, 周蕾, 等. CD109、DPC4/Smad4在食管鳞状细胞癌中的表达及其相关性[J]. 华中科技大学学报:医学版, 2017, 46(3):299-302. doi:10.3870/j.issn.1672-0741.2017.03.012. Song WQ, Yu L, Zhou L, et al. Expression of CD109 ,DPC4/Smad4 in Esophageal Squamous Cell Carcinoma and the Correlation[J]. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong, 2017, 46(3):299-302. doi:10.3870/j.issn.1672-0741.2017.03.012.
[20] Roland CL, Starker LF, Kang Y, et al. Loss of DPC4/SMAD4 expression in primary gastrointestinal neuroendocrine tumors is associated with cancer-related death after resection[J]. Surgery, 2017, 161(3):753-759. doi: 10.1016/j.surg.2016.09.002.
[21] Wang JD, Jin K, Chen XY, et al. Clinicopathological significance of SMAD4 loss in pancreatic ductal adenocarcinomas: a systematic review and meta-analysis[J]. Oncotarget, 2017, 8(10):16704-16711. doi: 10.18632/oncotarget.14335.
[22] Lee KT, Chang WT, Wang SN, et al. Expression of DPC4 /Smad4 gene in stone-containing intrahepatic bile duct[J]. J Surg Oncol, 2006, 94(4):338-343. doi: 10.1002/jso.20517.
[23] Chen TC, Jan YY, Yeh TS. K-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomy[J]. Ann Surg Oncol, 2012, 19(Suppl 3):S675-681.doi:10.1245/s10434-012-2224-7.
[24] Geradts J, Fong KM , Zimm erman PV , et al. Loss of Fhit expression in n on-small-cell lung cancer :correlation with molecular genetic abnormalities and clinicopathological features[J]. Br J Cancer, 2000, 82(7):1191-1197. doi: 10.1054/bjoc.1999.1062.
[25] Richter M, Zhang H. Receptor-targeted cancer therapy[J]. DNA Cell Biol, 2005, 24(5):271-282. doi: 10.1089/dna.2005.24.271.
[26] 郑军, 朱耀明, 邹声泉, 等. 原癌基因c-erbB-2在胆管癌细胞中的表达[J]. 肿瘤防治研究, 2007, 34(6):462-463. doi:10.3971/j.issn.1000-8578.2007.06.022. Zheng J, Zhu YM, Zou SQ, et al. Expression of Oncogene C-erbB-2 in the Cells of Cholangio Carcinoma[J]. Cancer Research on Prevention and Treatment, 2007, 34(6):462-463. doi:10.3971/j.issn.1000-8578.2007.06.022.
[27] Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma[J]. PLoS One, 2011, 6(1):e15640.doi:10.1371/journal.pone. 0015640.
[28] 朱垒, 黄飞舟, 聂晚频, 等. FIG-ROS融合基因在肝内胆管细胞癌中表达及其意义[J]. 中国普通外科杂志, 2015, 24(2):199-205. doi:10.3978/j.issn.1005-6947.2015.02.009. Zhu L, Huang FZ, Nie WP, et al. Expression of FIG-ROS fusion gene in intrahepatic cholangiocarcinoma and its significance[J]. Chinese Journal of General Surgery, 2015, 24(2):199-205. doi:10.3978/j.issn.1005-6947.2015.02.009.
[29] Deng L, Xiong P, Luo Y, et al. Association between IDH1/2 mutations and brain glioma grade[J]. Oncol Lett, 2018, 16(4):5405-5409. doi: 10.3892/ol.2018.9317.
[30] Lugowska I, Teterycz P, Mikula M, et al. IDH1/2 Mutations Predict Shorter Survival in Chondrosarcoma[J]. J Cancer, 2018, 9(6):998-1005. doi:10.7150/jca.22915.
[31] Farshidfar F, Zheng S, Gingras MC, et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles[J]. Cell Rep, 2017, 18(11):2780-2794. doi: 10.1016/j.celrep.2017.02.033.
[32] Sirirat T, Chuncharunee S, Nipaluk P, et al. Mutation Analysis of Isocitrate Dehydrogenase (IDH1/2) and DNA Methyltransferase 3A (DNMT3A) in Thai Patients with Newly Diagnosed Acute Myeloid Leukemia[J]. Asian Pac J Cancer Prev, 2017, 18(2):413-420. doi:10. 22034/APJCP.2017.18.2.413.
[33] 魏妙艳, 汤朝晖, 全志伟. 代谢在肝内胆管癌发病机制及临床诊治中的研究进展[J]. 世界华人消化杂志, 2017, 25(33):2929-2937. doi:10.11569/wcjd.v25.i33.2929. Wei MY, Tang ZH, Quan ZW. Intrahepatic cholangiocarcinoma: Role of metabolism in pathogenesis, clinical diagnosis, and treatment[J]. World Chinese Journal of Digestology, 2017, 25(33):2929-2937. doi:10.11569/wcjd.v25.i33.2929.
[34] Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma[J]. Hum Pathol, 2012, 43(10):1552-1558. doi: 10.1016/j.humpath.2011.12.007.
[35] Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing[J]. Oncologist, 2014, 19(3):235-242. doi: 10.1634/theoncologist.2013-0352.
[36] Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications[J]. PLoS One, 2014, 9(12):e115383. doi: 10.1371/journal.pone.0115383.
[37] Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets[J]. Ann Surg Oncol, 2014, 21(12):3827-3834. doi: 10.1245/s10434-014-3828-x.
[38] Shiao MS, Chiablaem K, Charoensawan V, et al. Emergence of Intrahepatic Cholangiocarcinoma: How High-Throughput Technologies Expedite the Solutions for a Rare Cancer Type[J]. Front Genet, 2018, 9:309. doi:10.3389/fgene. 2018.00309.
[39] 奚松阳, 房栋, 霍介格. 肝内胆管癌的分子靶向治疗进展[J]. 世界华人消化杂志, 2018, 26(29):1707-1716. doi: 10.11569/wcjd.v26.i29.1707. Xi SY, Fang D, Huo JG. Progress in molecular targeted therapy of intrahepatic cholangiocarcinoma [J]. World Chinese Journal of Digestology, 2018, 26(29):1707-1716. doi: 10.11569/wcjd.v26.i29.1707.
[40] Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications[J].Nat Rev Gastroenterol Hepatol, 2018, 15(3):137-151. doi: 10.1038/nrgastro.2017.169.
[41] 房锋, 宋天强. 微小RNA在肝细胞癌中的相关研究进展[J]. 中国普通外科杂志, 2018, 27(7):899-909. doi:10.3978/j.issn.1005- 6947.2018.07.015. Fang F, Song TQ. Research progress associated with microRNAs in hepatocellular carcinoma[J]. Chinese Journal of General Surgery, 2018,27(7):899-909. doi:10.3978/j.issn.1005-6947. 2018.07.015.
[42] Hu C, Huang F, Deng G, et al. miR-31 promotes oncogenesis in intrahepatic cholangi-ocarcinoma cells via the directsuppression of RASA1[J]. Exp Ther Med, 2013, 6(5):1265-1270. doi: 10.3892/etm.2013.1311.
[43] Chen L, Yan HX, Yang W, et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma[J]. J Hepatol, 2009, 50(2):358-369. doi: 10.1016/j.jhep.2008.09.015.
[44] Mott JL, Kurita S, Cazanave SC, et al. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc,hedgehog,and NFkappaB[J]. J Cell Biochem, 2010, 110(5):1155-1164. doi: 10.1002/jcb.22630.
[45] Mott JL, Kobayashi S, Bronk SF, et al. mir-29 regulates Mcl-1 protein expression and apoptosis[J]. Oncogene, 2007, 26(42):6133-6140. doi: 10.1038/sj.onc.1210436.
[46] Chusorn P, Namwat N, Loilome W, et al. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis[J].Tumor Biol, 2013, 34(3):1579-1588. doi: 10.1007/s13277-013-0688-0.
[47] Wang LJ, He CC, Sui X, et al. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN[J]. Oncotarget, 2015, 6(8):5932-5946. doi: 10.18632/oncotarget.3465.
[48] Liu Z, Jin ZY, Liu CH, et al. MicroRNA-21 regulates biological behavior by inducing EMT in human cholangiocarcinoma[J]. Int J Clin Exp Pathol, 2015, 8(5):4684-4694.
[49] Zeng B, Li Z, Chen R, et al. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3[J]. FEBS Lett, 2012, 586(19):3271-3278. doi: 10.1016/j.febslet.2012.06.049.
[50] Li B, Han Q, Zhu Y, et al. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting Twist [J]. FEBS J, 2012, 279(13):2393-2398. doi: 10.1111/j.1742-4658.2012.08618.x.
[51] Iwaki J, Kikuchi K, Mizuguchi Y, et al. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line[J]. PLoS One, 2013, 8(7):e69496. doi: 10.1371/journal.pone.0069496.
[52] Selaru FM, Olaru AV, Kan T, et al. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3[J]. Hepatology, 2009, 49(5):1595-1601. doi: 10.1002/hep.22838.
[53] Liu N, Jiang F, He TL, et al. The Roles of MicroRNA-122 Overexpression in Inhibiting Proliferation and Invasion and Stimulating Apoptosis of Human Cholangiocarcinoma Cells [J]. Sci Rep, 2015, 5:16566. doi: 10.1038/srep16566.
[54] Li J, Yao L, Li G, et al. miR-221 Promotes Epithelial-Mesenchymal Transition through Targeting PTEN and Forms a Positive Feedback Loop with β-catenin/c-Jun Signaling Pathway in Extra-Hepatic Cholangiocarcinoma[J]. PLoS One, 2015, 10(10):e0141168. doi: 10.1371/journal.pone.0141168.
[55] Qiu YH, Wei YP, Shen NJ, et al. miR-204 inhibits epithelial to mesenchymal transition by targeting slug in intrahepatic cholangiocarcinoma cells[J]. Cell Physiol Biochem, 2013, 32(5):1331-1341. doi: 10.1159/000354531.
[56] Morine Y, Shimada M, Iwahashi S, et al. Role of histone deacetylase expression in intrahepatic cholangiocarcinoma[J]. Surgery, 2012, 151(3):412-419. doi: 10.1016/j.surg.2011.07.038.
[57] 朱鹏. HDAC抑制剂诱导胆管癌细胞凋亡过程中aPKC表达的变化[D].武汉: 华中科技大学, 2013. Zhu P. HDAC Inhibitors Induce the Change of Expression of aPKC in Process of Apoptosis in Cholangiocarcinoma Cell[D]. Wuhan: Huazhong University of Science and Technology, 2013.
[58] 刘谋泽, 李浩, 张伟. 肝内胆管癌病理机制表观基因组学研究进展[J]. 中国临床药理学与治疗学, 2014, 19(11):1294-1298. Liu MZ, Li H, Zhang W. Epigenomic research progress on pathological mechanisms of intrahepatic cholangiocarcinoma[J]. Chinese Journal of Clinical Pharmacology and Therapeutics, 2014, 19(11):1294-1298.
[59] 单钰莹, 于茜, 陆才德. 胆道系统恶性肿瘤NCCN最新指南解读[J]. 现代实用医学, 2017, 29(7):843-846. doi:10.3969/j.issn.1671-0800.2017.07.002. Shan YY, Yu Q, Lu CD. Interpretation of the latest NCCN guidelines for bile duct malignant tumors[J]. Modern Practical Medicine, 2017, 29(7):843-846. doi:10.3969/j.issn.1671-0800.2017.07.002.
[60] Sia D, Tovar V, Moeini A, et al. Intrahepatic cholangiocarcinoma: pathogenesis and ration-ale for molecular therapies[J]. Oncogene, 2013, 32(41):4861-4870. doi: 10.1038/onc.2012.617.
[61] Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells[J]. Science, 2013, 340(6132):626-630. doi: 10.1126/science.1236062.
[62] Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation[J]. Science, 2013, 340(6132):622-626. doi: 10.1126/science.1234769.
[63] Chen JS, Hsu C, Chiang NJ, et al. A KRAS mutation statusstratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer[J]. Ann Oncol, 2015, 26(5):943-949. doi: 10.1093/annonc/mdv035.
[64] Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: Current status and emerging strategies[J]. World J Gastrointest Oncol, 2015, 7(11):338-346. doi: 10.4251/wjgo.v7.i11.338.
[65] Chaiteerakij R, Juran BD, Aboelsoud MM, et al. Association between variants in inflammation and cancer-associated genes and risk and survival of cholangiocarcinoma[J]. Cancer Med, 2015, 4(10):1599-1602. doi: 10.1002/cam4.501.
[66] 李勤裕, 施敏敏, 彭承宏. 埃罗替尼和塞来昔布抑制胆管癌生长和血管生成[J]. 外科理论与实践, 2012, 17(2):130-134. doi:10.3969/j.issn.1007-1096.2012.02.010. Li QY, Shi MM, Peng CH. Combination of erlotinib with celecoxib inhibits tumor growth and angiogenesis of cholangiocarcinoma[J]. Journal of Surgery Concepts & Practice, 2012, 17(2):130-134. doi:10.3969/j.issn.1007-1096.2012.02.010.
