胰腺实性假乳头状瘤(solid pseudopapillary tumor of the pancreas,SPTP)是一种少见的低度恶性肿瘤,组织来源尚未完全阐明,占胰腺肿瘤不到3%[1]。其好发于青年女性,临床症状不典型,术前确诊困难,确诊需病理及免疫组化分析。我院2007年1月—2018年12月间收治47例SPTP,现报告如下。
1 资料与方法
1.1 一般资料
本组47例患者,其中男11例,女36例,男女比例1:3.3;年龄15~65岁,平均(32±15)岁;有临床症状者30例(63.8%),其中腹痛、腹胀21例(44.7%),恶心、呕吐8例(17.0%),腰背痛2例(4.3%),乏力1例(2.1%),因外伤致肿瘤破裂出血就诊1例(2.1%);17例(36.2%)为体检发现胰腺占位。首发症状至就诊的时间为3 d至7年,平均18.7个月。肿瘤均为单发,位于胰头部16例,胰颈部8例,胰体尾部23例。肿瘤最大直径为2.2~13 cm,平均(5.8±2.7)cm。
1.2 辅助检查
所有患者血清CEA、CA19-9和CA125水平均正常。术前通过CT和/或MRI检查初步诊断为SPTP者21例(44.7%,21/47);考虑为其他类型肿瘤者13例(27.7%),其中胰腺囊腺瘤7例,胰母细胞瘤2例,间质瘤、假性囊肿、囊腺癌、神经内分泌瘤各1例;余13例性质不确定。6例术前行超声内镜引导下细针穿刺活检术(EUS-FNA),其中仅1例(16.7%)确诊为SPTP。
1.3 手术情况
胰十二指肠切除术10例(其中机器人辅助1例),胰体尾联合脾脏切除术12例(腹腔镜手术3例),保留脾脏的胰体尾切除术8例(腹腔镜手术2例),胰腺中段切除术5例(腹腔镜手术1例),肿瘤局部切除术10例(腹腔镜手术1例),保留十二指肠胰头切除术1例,胰体尾联合脾、左肾及左肾上腺切除+下腔静脉切开取栓术1例。
2 结 果
2.1 术后情况
术后并发症按照按中华医学会外科学分会胰腺外科学组制定的胰腺术后外科常见并发症诊断标准[2],本组9例(19.1%,9/47)术后发生并发症,其中3例发生2种以上并发症。并发症包括:B级胰瘘5例(10.6%,5/47),胃排空延迟4例(8.5%,4/47),胆瘘、腹腔出血、腹腔积液者各1例(2.1%,1/47)。按Clavien-Dindo并发症分级:I级1例,II级6例,IIIa、IIIb级各1例。合并胰瘘或胆瘘患者给予腹腔持续冲洗引流、抑制胰酶分泌和抗感染等治疗;胃排空延迟者予以胃镜下置空肠营养管行肠内营养支持,同时予以胃复安、红霉素等促进胃动力治疗;腹腔出血者则给予止血、输血等治疗。本组所有出现并发症患者均经保守治疗后痊愈。无再手术和手术相关死亡。术后住院时间为7~42 d,平均(11.6±6.1)d。
2.2 病理及免疫组化结果
肿瘤呈囊性8例,实性8例,囊实性31例;包膜完整者35例。术中行快速冷冻病理检查者9例,其中7例考虑为SPTP,2例考虑为神经内分泌肿瘤。术后病理2例见恶性特征:1例肿瘤侵犯脾脏,1例下腔静脉、左肾静脉内见癌栓,左肾上腺受侵犯。所有患者肿瘤切缘及淋巴结均阴性。
肿瘤免疫组织化学检查结果:神经内肽酶(CD10)阳性91.4%(32/35),神经细胞黏附因子(CD56)阳性100%(33/33),波形蛋白(vimentin)阳性92.3%(36/39),角蛋白(CK)阳性66.7%(24/36),α1-抗胰蛋白酶(α1-AT)阳性83.3%(20/24),α1-抗胰糜蛋白酶(α1-ACT)阳性90.5%(19/21),神经特异性烯醇化酶(NSE)阳性97.3%(36/37),嗜铬粒蛋白(C g A)阳性13.2%(5/38),突触素(S y n)阳性62.2%(23/37),S 100(S100蛋白)阳性35.0%(7/20),β-catenin阳性87.5%(7/8),孕激素受体(P R)阳性100%(38/38),雌激素受体(E R)阳性0(0/25),Ki-67<1.5%阳性55.6%(20/36),≥1.5%阳性44.4%(16/36),癌胚抗原(CEA)阳性20.0%(2/10),上皮膜抗原(EMA)阳性5.0%(1/20),胰岛素(insulin)阳性0(0/11),胰高血糖素(glucagon)阳性0(0/10),CD99蛋白(CD99)阳性60.0%(6/10)(表1)。
表1 免疫组化检测结果
Table1 Results of immunohistochemical examination
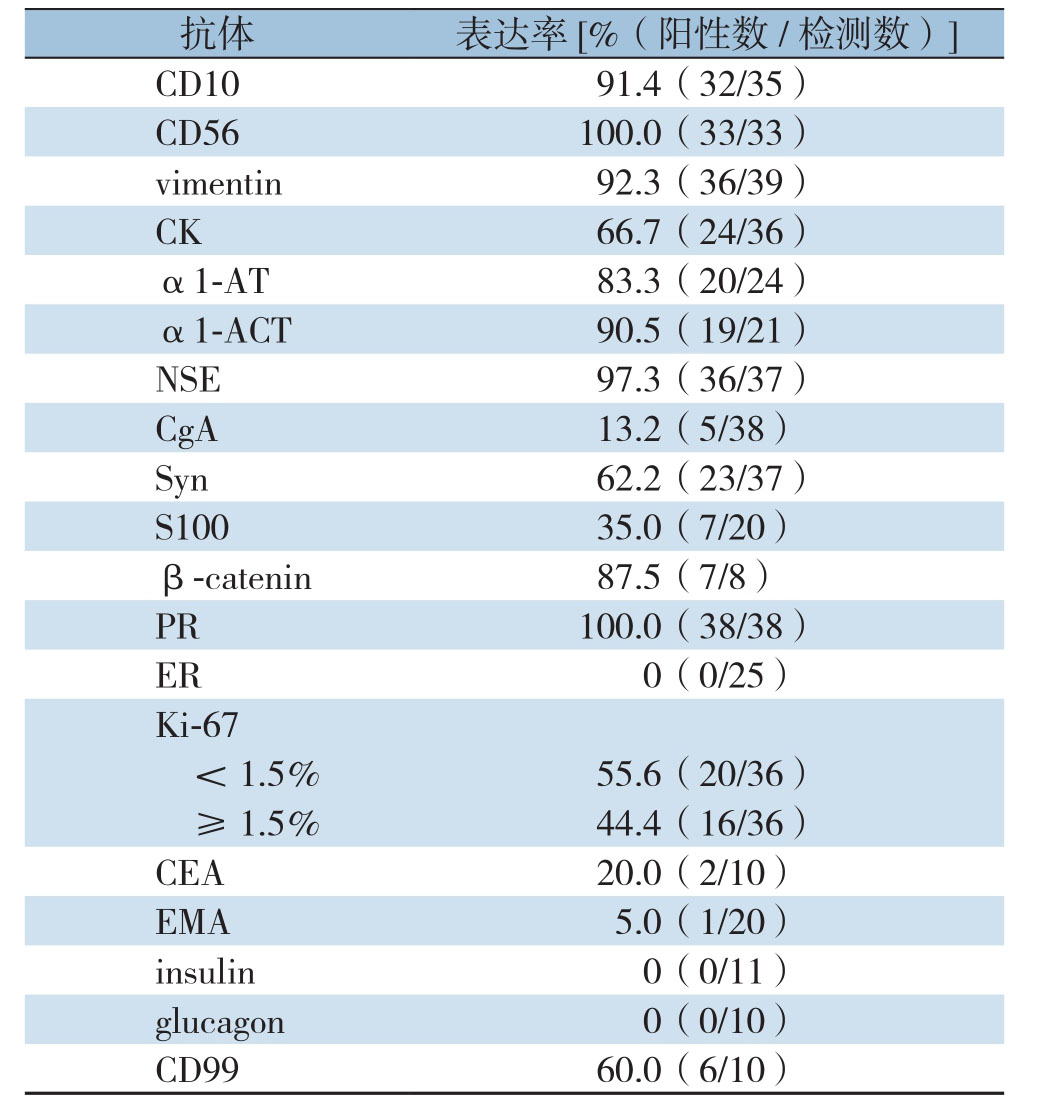

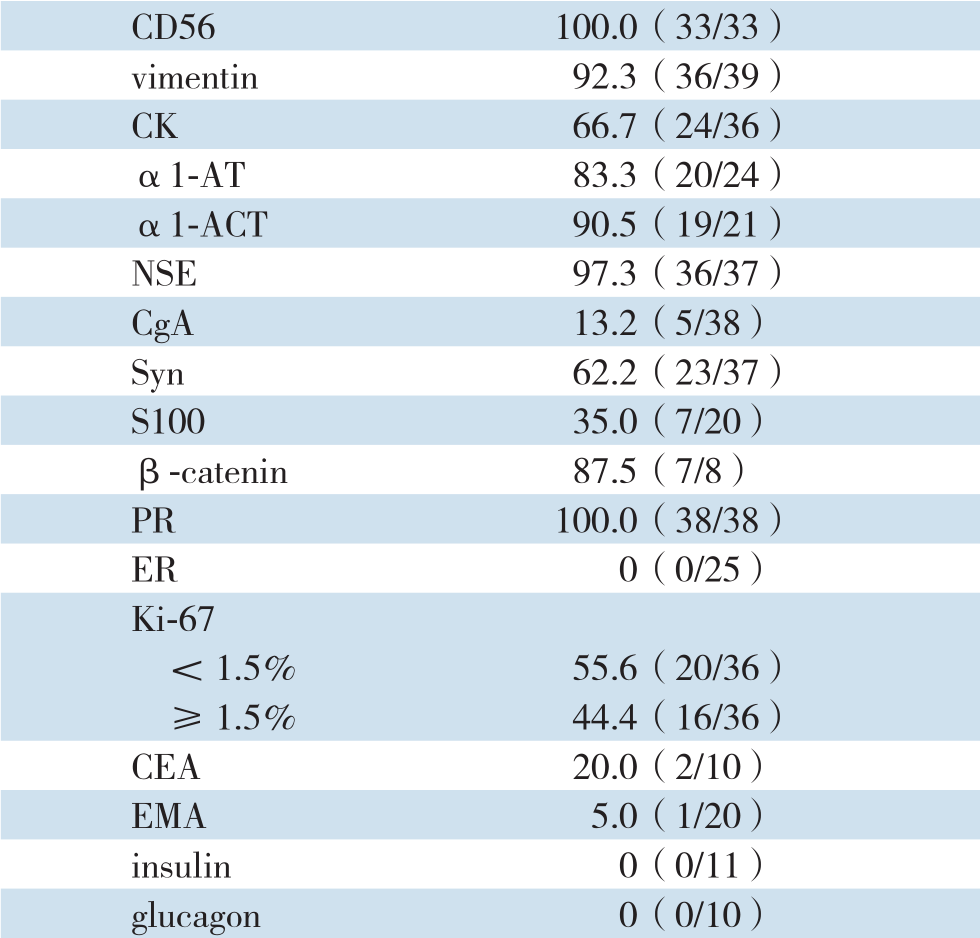 C D10 91.4(32/35)CD99 60.0(6/10)
C D10 91.4(32/35)CD99 60.0(6/10)
2.3 随访情况
术后随访至2018年12月,43例获随访,4例失访,随访时间2~141个月,平均(41.7±31.4)个月。所有获随访的患者均存活,其中血管内见癌栓、肾上腺受侵犯患者于术后第3个月复发,已行吉西他滨+替吉奥方案化疗6个周期,目前为单药口服替吉奥化疗且疾病稳定。其余患者随访期内无肿瘤复发及转移。
3 讨 论
SPTP由Frantz[3]在1959年首次报道,世界卫生组织(WHO)1996年正式将该病命名为“胰腺实性假乳头状瘤”,2000年将其分为两类:胰腺实性假乳头状瘤(交界性)和胰腺实性假乳头状癌,并于2010年将所有SPTP定义为低度恶性肿瘤[4-6]。SPTP发病率低,占胰腺肿瘤的1%~3%;主要发生在青年女性,男女比例约为1:7.2,平均年龄28.5岁[7]。本组男女比1:3.3,平均年龄32岁。
SPTP的临床表现无特异性。患者多表现为腹痛或腹部不适;部分患者为体检发现腹部肿块;也有极少数患者因肿瘤包膜破裂、腹腔积血和继发感染等急性症状就诊。SPTP患者很少出现梗阻性黄疸,即使位于胰头,也极少伴胰胆管扩张,这可能与肿瘤外生性生长的特性有关[1,7]。SPTP的临床表现主要与肿瘤的生长部位、大小有关,症状也常由肿瘤压迫、侵犯邻近组织器官所引起。无症状患者的肿瘤通常较有症状患者小,而在影像学上的表现基本相似[8]。SPTP患者的实验室检查、肿瘤标志物一般都正常;术前诊断主要依据影像学检查,但有时难与其他胰腺肿瘤鉴别,如囊腺瘤、非功能性神经内分泌肿瘤、假性囊肿等。本组术前影像学检查仅44.7%初步诊断为SPTP。超声或CT引导下细针穿刺病理学检查,可获取病理资料,是术前确诊本病的唯一方法。EUS-FNA对SPTP术前诊断率约为69.5%[7];而在联合CT或CT和EUS时,诊断率可达82.4%[9]。
SPTP的确诊最终依靠术后病理及免疫组化检查。SPTP镜下表现为肿块由实性区、假乳头区、囊性区混合而成,以及典型的肿瘤细胞围绕纤维血管轴心生长形成分支状假乳头结构或假菊形团结构[10]。在免疫组化中,一些免疫蛋白被用于预测恶性程度及复发指标,如Ki-67,然而其预测作用仍存在争议。WHO在2000年关于SPTP的分类中,Ki-67≥1.5%被认为是恶性的重要指标[5]。Yang等[11]和Yu等[12]认为Ki-67的阳性指数与肿瘤恶性行为和不良预后相关,Suzuki等[13]则发现Ki-67指数较高的肿瘤未显示恶性特征。本组Ki-67≥1.5%表达率为44.4%,包括2例病理表现为恶性特征者。SPTP的免疫组化显示,几乎所有病例都可见PR阳性,而ER阳性非常罕见,这似乎与女性多发并不完全相符;而Tognarini等[14]和Kurokawa等[15]的研究显示雌激素与SPTP的生长相关。本组患者PR全部呈阳性表达,ER无表达。
SPTP的首选治疗为手术切除,手术切除率可达95%[7]。根据肿瘤好发部位,常见的术式为:胰体尾切除或联合脾脏切除术,其次是胰十二指肠切除术和肿瘤局部切除术。腹腔镜手术治疗SPTP是安全、可行的,同样可获得较好的术后效果[16-17]。肿瘤属于低度恶性,手术要求R0切除,且尽可能选择保留器官功能的胰腺切除术[18]。由于其惰性的生物学行为,即使肿瘤对周围组织浸润或远处转移,均不视为手术禁忌证,可给予标准化手术或联合脏器切除[12,19]。SPTP局部侵袭可见于十二指肠、胃、脾脏、血管等,转移则多发生在肝脏,而淋巴结转移少见[20],故一般情况下不主张淋巴结清扫。本组所有送检淋巴结组织,病理均为反应性增生。目前对于放化疗治疗SPTP的方案及临床疗效尚缺乏共识,仅有少数病例报道[21-22]。
WHO在2010年[6]将SPTP分类为低度恶性肿瘤,且存在多发转移、胰周侵犯、淋巴结转移时定义为复发的高危因素;但SPTP无论有无恶性行为,术后均可能复发、转移等。手术切除是影响预后的最主要因素;而对其的复发预测因素,目前仍未达成统一观点[23]。Kang等[24]认为肿瘤大小>8 cm、显微镜下恶性肿瘤特征和肿瘤IV期是肿瘤复发的重要危险因素。而Jutric等[25]则认为性别、年龄、肿瘤大小、淋巴结转移的存在、手术切缘阳性和远处转移的存在都不是SPTP患者手术治疗后生存的显著预测因子。
SPTP总体预后较好,其恶性程度低,只有9%~15%患者出现局部浸润或转移[26]。Law等[7]报道的1952例随访患者中,SPTP的复发率约4.4%,平均复发时间50.5个月,因SPTP死亡患者仅29例(1.5%)。Papavramidis等[1]报道的718例患者,2、5年总生存率分别为97%、95%,在行完整肿瘤切除术后的患者均获得了较长的生存期。Yu等[20]统计的国内553例患者5年生存率为96.9%,复发、转移率仅2.3%。Jutric等[25]回顾分析了340例患者的临床资料,8年生存率为85%,并认为接受任何类型手术切除的患者均获得了长期生存。Hao等[27]报道了59例病理显示恶性行为患者,在完整切除肿瘤后仍可获得10年以上的生存期。在肿瘤复发、转移患者中,经再次手术后仍可获得较长的生存期[25-26]。本组术后平均随访(41.7±31.4)个月,所有获得随访的患者均存活,但有1例术后出现复发。
总之,SPTP是一种好发于年轻女性的潜在低度恶性肿瘤。其临床表现无特异性,确诊有赖于特征性的病理组织表现及免疫组化检查,手术切除是首选治疗方法。尽管其总体预后良好,但术后仍可能复发及转移,因此有必要对所有患者长期随访。
[1]Papavramidis T,Papavramidis S.Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature[J].J Am Coll Surg,2005,200(6):965-972.doi: 10.1016/j.jamcollsurg.2005.02.011.
[2]中华医学会外科学分会胰腺外科学组,中国研究型医院学会胰腺病专业委员会,中华外科杂志编辑部.胰腺术后外科常见并发症诊治及预防的专家共识(2017)[J].中华外科杂志,2017,55(5):328-334.doi:10.3760/cma.j.issn.0529-5815.2017.05.003.Study Group of Pancreatic Surgery in Chinese Socie,Pancreatic Disease Committee of Chinese Research Hospital,Editorial Board of Chinese Journal of Surgery.A consensus statement on the diagnosis,treatment,and prevention of common complications after pancreatic surgery (2017)[J].Chinese Journal of Surgery,2017,55(5):328-334.doi:10.3760/cma.j.issn.0529-5815.2017.05.003.
[3]Frantz VK.Tumors of the pancreas[A]//Atlas of tumor pathology[M].Washington: Armed Forces Institute of Pathology,1959:32-33.
[4]Klöppel G,Heitz PU,Capella C,et al.Pathology and nomenclature of human gastrointestinal neuroendocrine (carcinoid) tumors and related lesions[J].World J Surg,1996,20(2):132-141.
[5]Klöppel G,Lüttges J,Klimstra D,et al.Solid-pseudopapillary neoplasm[A]//Hamilton SR,Aaltonen LA,editors.WHO classification of tumours.Pathlogy and genetics of tumors of of the digestive system[M].Lyon: International Agency for Research on Cancer,2000:246-248.
[6]Bosman FT,Carneiro F,Hruban RH,et al.WHO classification of tumours of the digestive system[M].4th ed.Lyon: International Agency for Research on Cancer,2010:327-330.
[7]Law JK,Ahmed A,Singh VK,et al.A systematic review of solid-pseudopapillary neoplasms: are these rare lesions?[J].Pancreas,2014,43(3):331-337.doi:10.1097/MPA.0000000000000061.
[8]Hu S,Zhang H,Wang X ,et al.Asymptomatic versus symptomatic solid pseudopapillary tumors of the pancreas: clinical and MDCT manifestations[J].Cancer Imaging,2019,19(1):13.doi: 10.1186/s40644-019-0198-4.
[9]Law JK,Stoita A,Wever W,et al.Endoscopic ultrasound-guided fine needle aspiration improves the pre-operative diagnostic yield of solid-pseudopapillary neoplasm of the pancreas: an international multicenter case series (with video)[J].Surg Endosc,2014,28(9):2592-2598.doi:10.1007/s00464-014-3508-8.
[10]Dinarvand P,Lai J.Solid Pseudopapillary Neoplasm of the Pancreas: A Rare Entity With Unique Features[J].Arch Pathol Lab Med,2017,141(7):990-995.doi:10.5858/arpa.2016-0322-RS.
[11]Yang F,Yu X,Bao Y,et al.Prognostic value of Ki-67 in solid pseudopapillary tumor of the pancreas: Huashan experience and systematic review of the literature[J].Surgery,2016,159(4):1023-1031.doi:10.1016/j.surg.2015.10.018.
[12]Yu P,Cheng X,Du Y,et al.Solid Pseudopapillary Neoplasms of the Pancreas: a 19-Year Multicenter Experience in China[J].J Gastrointest Surg,2015,19(8):1433-1440.doi: 10.1007/s11605-015-2862-8.
[13]Suzuki S,Hatori T,Furukawa T,et al.Clinical and Pathological Features of Solid Pseudopapillary Neoplasms of the Pancreas at a Single Institution[J].Dig Surg,2014,31(2):143-150.doi:10.1159/000363420.
[14]Tognarini I,Tonelli F,Nesi G,et al.In vitro effects of oestrogens,antioestrogens and SERMs on pancreatic solid pseudopapillary neoplasm-derived primary cell culture[J].Cell Oncol,2010,32(5/6):331-343.doi: 10.3233/CLO-2010-0522.
[15]Kurokawa S,Hirabayashi K,Hadano A,et al.Do Solid Pseudopapillary Neoplasms Shrink After Menopause?: Review of the Literature[J].Pancreas,2015,44(6):998-999.doi: 10.1097/MPA.0000000000000358.
[16]Chen XM,Zhang Y,Sun DL.Laparoscopic central pancreatectomy for solid pseudopapillary tumors of the pancreas: our experience with ten cases[J].World J Surg Oncol,2014,12:312.doi:10.1186/1477-7819-12-312.
[17]Afridi SA,Kazaryan AM,Marangos IP,et al.Laparoscopic surgery for solid pseudopapillary tumor of the pancreas [J].JSLS,2014,18(2):236-242.doi: 10.4293/108680813X13753907291837.
[18]肖卫东,林生荣,吴安涛,等.保留器官的胰腺切除术治疗胰腺良性或低度恶性肿瘤:单中心66例报告[J].中国普通外科杂志,2018,27(3):284-288.doi:10.3978/j.issn.1005-6947.2018.03.003.Xiao WD,Lin SR,Wu AT,et al.Organ preserving pancreatectomy for pancreatic benign or low-grade malignant tumor: a report of 66 cases in a single institution[J].Chinese Journal of General Surgery,2018,27(3):284-288.doi: 10.3978/j.issn.1005-6947.2018.03.003.
[19]Naar L,Spanomichou DA,Mastoraki A,et al.Solid Pseudopapillary Neoplasms of the Pancreas: A Surgical and Genetic Enigma[J].World J Surg,2017,41(7):1871-1881.doi: 10.1007/s00268-017-3921-y.
[20]Yu PF,Hu ZH,Wang XB,et al.Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature[J].World J Gastroenterol,2010,16(10):1209-1214.
[21]Tajima H,Takamura H,Kitagawa H,et al.Multiple liver metastases of pancreatic solid pseudopapillary tumor treated with resection following chemotherapy and transcatheter arterial embolization:A case report[J].Oncol Lett,2015,9(4):1733-1738.doi:10.3892/ol.2015.2967.
[22]Krug S,Bartsch DK,Schober M,et al.Successful selective internal radiotherapy (SIRT) in a patient with a malignant solid pseudopapillary pancreatic neoplasm (SPN)[J].Pancreatology,2012,12(5):423-427.doi: 10.1016/j.pan.2012.07.014.
[23]You L,Yang F,Fu DL,Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas[J].World J Gastrointest Oncol,2018,10(7):184-193.doi: 10.4251/wjgo.v10.i7.184.
[24]Kang CM,Choi SH,Kim SC,et al.Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea[J].Ann Surg,2014,260(2):348-355.doi:10.1097/SLA.0000000000000583.
[25]Jutric Z,Rozenfeld Y,Grendar J,et al.Analysis of 340 Patients with Solid Pseudopapillary Tumors of the Pancreas: A Closer Look at Patients with Metastatic Disease [J].Ann Surg Oncol,2017,24(7):2015-2022.doi: 10.1245/s10434-017-5772-z.
[26]Zhang H,Wang W,Yu S,et al.The prognosis and clinical characteristics of advanced (malignant) solid pseudopapillary neoplasm of the pancreas[J].Tumour Biol,2016,37(4):5347-5353.doi: 10.1007/s13277-015-4371-5.
[27]Hao EIU,Hwang HK,Yoon DS,et al.Aggressiveness of solid pseudopapillary neoplasm of the pancreas: A literature review and meta-analysis[J].Medicine (Baltimore),2018,97(49):e13147.doi:10.1097/MD.0000000000013147.
