胃肠间质瘤(gastrointestinal stromal tumor,GIST)起源于Cajal细胞,是胃肠道最常见的间叶源性肿瘤,发病率约11~19/100万[1-2],占胃肠道恶性肿瘤的1%~3%[3-4]。随着靶向药物伊马替尼的出现,GIST的治疗模式发生了巨大的变化,由原来的单纯手术完整切除到现在的以手术治疗为主,靶向药物酪氨酸激酶抑制剂为重要的辅助治疗手段。
近年来,临床工作中诊治GIST患者越来越多,GIST已成为胃肠外科常见病之一。本研究通过对笔者医院2012年1月—2018年8月期间诊治的原发GIST患者的临床病理资料进行回顾性分析,探讨GIST临床病理特征及预后影响因素,以期为GIST的诊治提供参考。
1 资料与方法
1.1 一般资料
回顾性分析中南大学湘雅医院2012年1月—2018年8月期间诊治的326例GIST患者的临床病理资料。其中12例(3.7%)为 消化道恶性肿瘤伴发GIST,其余314例(96.3%)为原发GIST。原发GIST复发风险根据2008年改良美国国立卫生研究院(national institutes of health,NIH)危险度分级标准。纳入标准:病理组织学和免疫组织化学检查确诊为GIST。排除标准:合并有其他恶性肿瘤或恶性肿瘤病史者;临床病理学资料不完整者。
1.2 随访
通过门诊复查、电话或微信等方式进行随访,了解患者的服药情况、不良反应、肿瘤有无复发和生存情况。随访时间截止至2018年10月。
1.3 统计学处理
应用SPSS 25.0统计软件进行分析,数据以例数(百分率)[n(%)]表示,用Kaplan-Meier 法进行生存分析。
2 结 果
2.1 患者临床病理资料及治疗情况
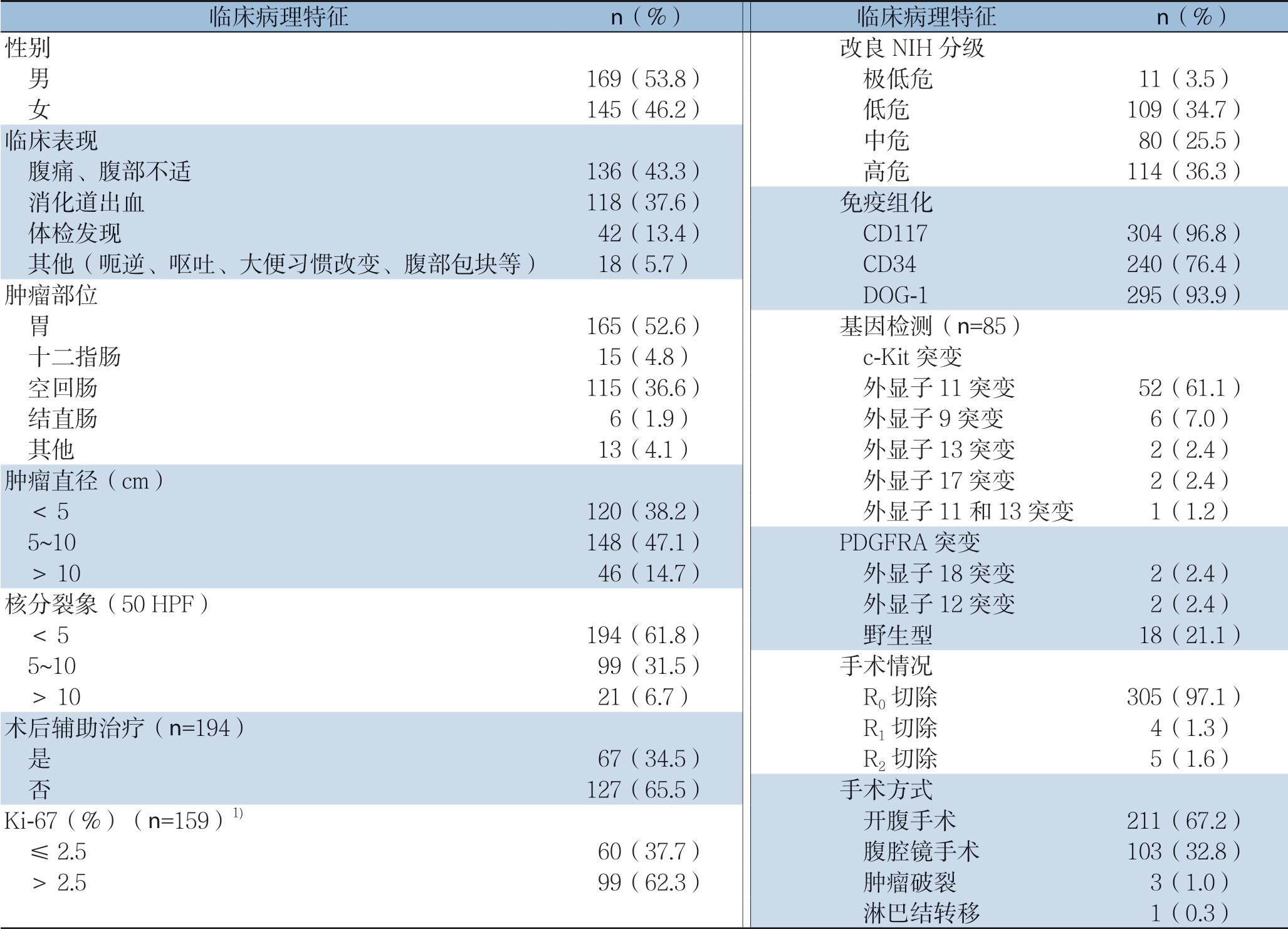
314例原发GIST患者中,男性169例(53.8%),女性145例(46.2%),中位年龄为55(24~86)岁。肿瘤直径6(1~34)cm。原发部位位于胃者 165例(52.6%),十二指肠15例(4.8%),空回肠115例(36.6%),结直肠6例(1.9%),其他部位(肠系膜、腹膜后、盆腔等)13例(4.1%)。根据NIH 2008改良版分级,极低危患者11例(3.5%),低危患者109例(34.7%),中危患者80例(25.5%),高危患者114例(36.3%)。314例患者均接受手术治疗,其中R0切除305例(97.1%),R1切除4例(1.3%),R2切除5例(1.6%)。194例中高危患者中,67例(34.5%)患者术后接受伊马替尼辅助治疗。中位治疗时间为26(7~56)个月。手术情况、核分裂象、基因突变等详见表1。
表1 314例原发GIST患者的基本资料
Table 1 The general data of the 314 patients with primary GIST
注:1)参考以往研究[5]
Note: 1) Based on previous study[5]
临床病理特征 n(%) 临床病理特征 n(%)性别 改良NIH分级 男 169(53.8) 极低危 11(3.5) 女 145(46.2) 低危 109(34.7)临床表现 中危 80(25.5) 腹痛、腹部不适 136(43.3) 高危 114(36.3) 消化道出血 118(37.6) 免疫组化 体检发现 42(13.4) CD117 304(96.8) 其他(呃逆、呕吐、大便习惯改变、腹部包块等) 18(5.7) CD34 240(76.4)肿瘤部位 DOG-1 295(93.9) 胃 165(52.6) 基因检测(n=85) 十二指肠 15(4.8) c-Kit突变 空回肠 115(36.6) 外显子11突变 52(61.1) 结直肠 6(1.9) 外显子9突变 6(7.0) 其他 13(4.1) 外显子13突变 2(2.4)肿瘤直径(cm) 外显子17突变 2(2.4) <5 120(38.2) 外显子11和13突变 1(1.2) 5~10 148(47.1) PDGFRA突变 >10 46(14.7) 外显子18突变 2(2.4)核分裂象(50 HPF) 外显子12突变 2(2.4) <5 194(61.8) 野生型 18(21.1) 5~10 99(31.5) 手术情况 >10 21(6.7) R0切除 305(97.1)术后辅助治疗(n=194) R1切除 4(1.3) 是 67(34.5) R2切除 5(1.6) 否 127(65.5) 手术方式Ki-67(%)(n=159)1) 开腹手术 211(67.2) ≤2.5 60(37.7) 腹腔镜手术 103(32.8) >2.5 99(62.3) 肿瘤破裂 3(1.0) 淋巴结转移 1(0.3)
2.2 随访情况
314例患者中,268例(85.4%)获得有效随访,失访46例(14.6%),中位随访时间为37(2~69)个月。1、3、5年总体无复发生存率分别为97.0%、92.6%、81.7%(图1A);1、3、5年总体总存活率分别为99.4%、95.2%、88.2%(图1B)。根据危险度分级,极低危、低危、中危及高危患者其5年无复发存活率分别为100.0%、93.3%、79.1%、64.4%(图2A);5年总存活率分别为100.0%、94.1%、91.7%、74.9%(图2B)。194例中高危GIST患者中,67例术后接受伊马替尼辅助治疗者及127例术后未接受伊马替尼辅助治疗者5年无复发存活率分别为73.8%、65.2%(χ2=4.910,P=0.027),有统计学差异。5年总存活率分别为87.5%、71.6%(χ2=3.160,P=0.075),无统计学差异。
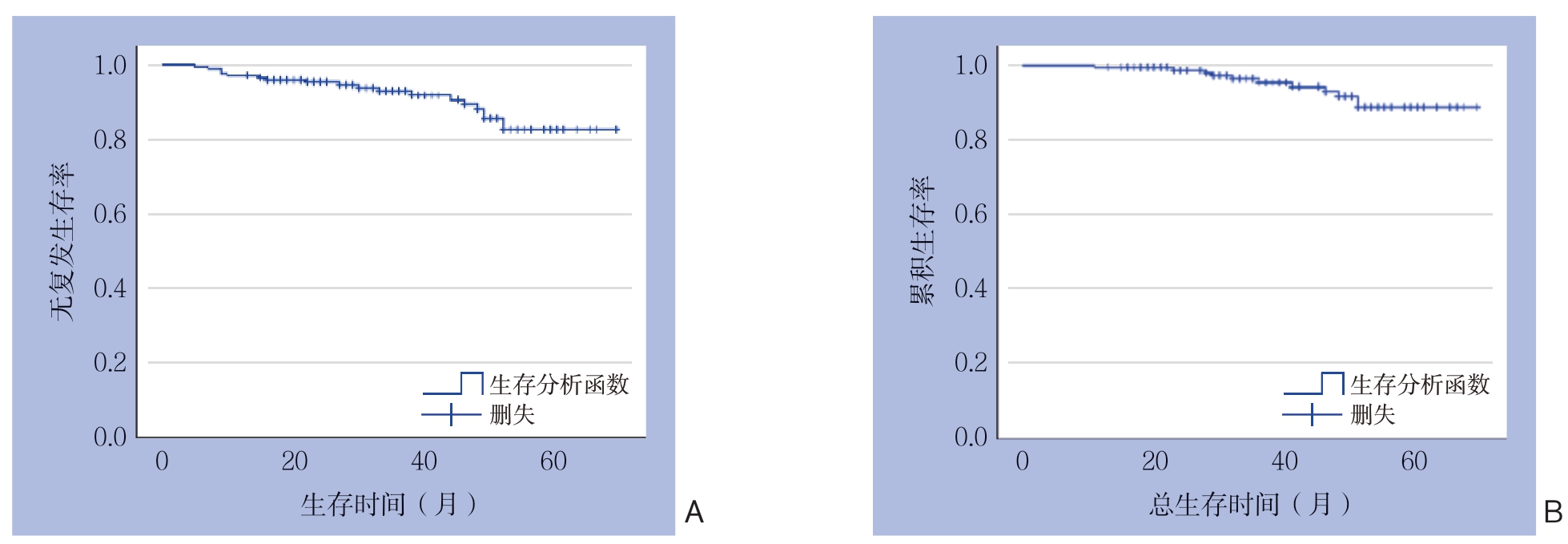
图1 全组患者生存曲线
Figure 1 Survival curves for the entire groups of patients
A:无复发生存曲线;B:总体生存曲线
A: Relapse-free survival curve; B: Overall survival curve
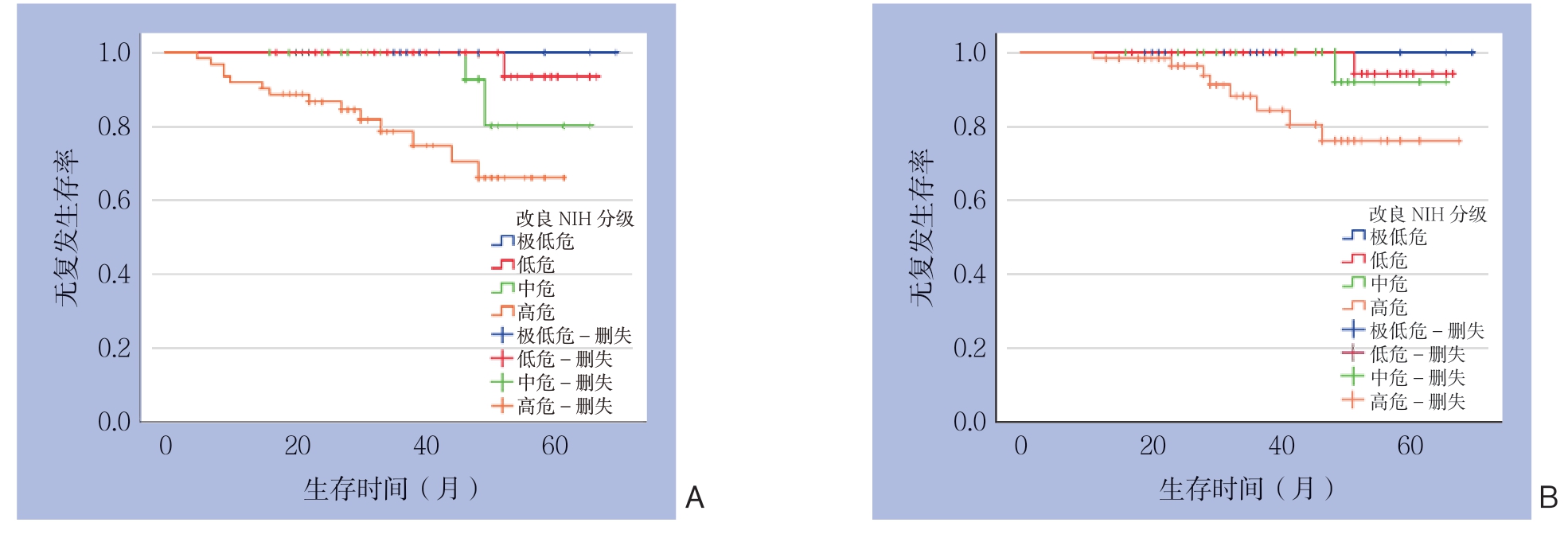
图2 不同危险度分级患者生存曲线
Figure 2 Survival curves for patients with different risk degrees
A:无复发生存曲线;B:总体生存曲线
A: Relapse-free survival curve; B: Overall survival curve
3 讨 论
GIST可发生于消化道的任何部位,也可发生于网膜、肠系膜、腹膜和腹盆腔等消化道外[6],但最常见于胃(约60%),其次是小肠(25%~30%),少数发生于结直肠(约5%)、食管及其他部位[7]。本组病例资料结果显示,胃和小肠是GIST最多见的发病部位,与以往文献[8]报道相似。GIST一般高发于中老年人,好发年龄为50~70岁[9]。本研究中,发病中位年龄为55岁,50岁及以上者占比67.2%,和上述文献报道相一致。
有临床症状的GIST大约占70%左右,20%无临床症状的GIST是偶然发现的,10%的GIST病灶仅在尸检时才被发现。GIST患者症状和体征都无特异性,主要和肿瘤大小、部位及生长方式有关。本组数据中,以表现为腹痛、腹部不适者136例(43.3%)及消化道出血症状者118例(37.6%)最多见,与文献[8,10-11]报道相似。CT等影像学检查对GIST有重要诊断价值,但有些GIST向腔外生长,特别是巨大GIST,容易推压、侵犯临近组织器官,当肿瘤与临近组织器官关系密切时,诊断存在误诊可能[12]。同时由于GIST血供丰富,活体组织病理学检查可能引起肿瘤的破溃、出血和增加肿瘤播散的危险性,应当慎重施行。而对于术前评估可以完整切除并且不影响相应脏器功能者不推荐术前活检,可以直接行手术切除[13]。
对于原发局限性GIST,外科手术完整切除仍是最重要的治疗手段[14],具体手术方式及切除范围根据肿瘤的生长部位、大小以及肿瘤与临近组织器官关系而定。GIST的淋巴结转移率很低,本研究仅发现1例(0.3%)发生淋巴结转移,故常规不必行淋巴结清扫。手术应遵循无瘤原则和尽可能做到镜下切缘阴性[15]。本组314例患者行R0切除305例(97.1%),R1切除4例(1.3%),R2切除5例(1.6%),术中3例(1.0%)患者出现肿瘤破裂。有些患者未能行R0切除,原因主要有肿瘤巨大合并有出血梗阻等并发症,术中发现肿瘤侵犯周围组织或者有远处转移无法行R0切除。首次手术完整切除和术中避免肿瘤破裂是GIST外科治疗成功的关键[16]。此外,对于术前评估难以完整切除,或肿瘤过大,术中容易出血和破裂、或特殊部位的肿瘤,如胃食管结合部、十二指肠及低位直肠等,以及手术易损害重要脏器功能者,在取得活检明确病理诊断后可先行伊马替尼术前治疗再考虑外科手术治疗[17-19]。
Z9001临床研究以安慰剂组或随访组作为对照,通过对比1年辅助治疗组和对照组发现伊马替尼可显著改善有复发风险的GIST患者预后[20]。随后SSGXV Ⅲ期随机研究[21]发现对高复发风险的患者给予术后伊马替尼治疗36个月较12个月能明显改善其无复发存活率和总存活率。因此,对于中高危患者应行伊马替尼辅助治疗,2017年版中国胃肠间质瘤诊断治疗共识推荐胃来源中危GIST应辅助治疗1年;非胃来源中危GIST应辅助治疗3年;高危GIST患者辅助治疗至少3年;而对于发生肿瘤破裂者,可以考虑延长辅助治疗时间[22]。本研究中,中高危GIST患者共194例,而行伊马替尼辅助治疗者仅67例(34.5%),比例偏低,原因主要有家庭经济能力无法承受伊马替尼辅助治疗费用和对该疾病认识不够而拒绝行伊马替尼治疗。
美国国家综合癌症网络(NCCN)指南推荐对GIST患者常规进行基因检测。2017年版中国胃肠间质瘤诊断治疗共识也指出,对于原发可切除GIST,术后拟行伊马替尼辅助治疗者应行基因检测。基因突变类型对指导酪氨酸激酶抑制剂治疗、判断药物疗效及预测预后等方面均有重要作用[23]。因此,建议GIST患者行基因检测,明确有无基因突变及突变类型。本组314例患者中,行基因检测者85例,检测率为27.1%。其中c-Kit外显子11突变最常见,为52例(61.1%),其余依次为野生型18例(21.1%),c-Kit外显子9突变6例(7.0%),c-Kit外显子13及17突变各2例(2.4%),PDGFRA外显子12及18突变各2例(2.4%),c-Kit外显子11及13同时突变1例(1.2%),与以往报道[24-26]相似。
在临床工作中,笔者发现有些高危GIST患者在术后伊马替尼治疗3年停药后仍会出现复发转移的情况,而对于此类患者,莫非最终只能选择不停药?作为一种毒副反应相对较小的靶向药物,伊马替尼的治疗安全性的确很高,但一味延长伊马替尼治疗时间势必会导致毒副反应的增加,而且延长伊马替尼治疗时间是否会增加耐药情况目前尚不可知。Lin等[27]的研究收集了234例行R0切除并接受了伊马替尼术后辅助治疗的GIST患者,并分析用药时间对远期疗效的影响。该研究发现随着伊马替尼治疗时间的延长,高危GIST患者的远期预后有所改善,因此建议高危GIST患者接受至少5年的伊马替尼治疗。笔者认为需要针对不同的高危GIST患者量身定制个体化治疗方案,比如肿瘤>10 cm,核分裂像>10/50 HPF或者术中肿瘤破裂者,可适当延长伊马替尼辅助治疗时间,并且在规范辅助治疗的同时,还需进行严密系统地随访。
综上所述,胃和小肠是GIST最常见的发病部位,原发GIST外科手术完整切除并根据危险度分级予以伊马替尼辅助治疗预后较好,但需针对不同的高危GIST患者量身定制个体化治疗方案,并且在规范辅助治疗的同时,还需进行严密系统地随访。
[1]Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing[J]. PLoS One, 2011, 6(8):e20294. doi: 10.1371/journal.pone.0020294.
[2]Mazzola P, Spitale A, Banf i S, et al. Epidemiology and molecular biology of gastrointestinal stromal tumors (GISTs): a populationbased study in the South of Switzerland, 1999-2005[J]. Histol Histopathol, 2008, 23(11):1379-1386. doi: 10.14670/HH-23.1379.
[3]Cioffi A, Maki RG. GI Stromal Tumors: 15 Years of Lessons From a Rare Cancer[J]. J Clin Oncol, 2015, 33(16):1849-1854. doi: 10.1200/JCO.2014.59.7344.
[4]von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors[J]. J Clin Oncol, 2018, 36(2):136-143. doi: 10.1200/JCO.2017.74.9705.
[5]朱从波, 廖国庆, 赵丁民. Ki-67对胃肠道间质瘤预后的评估价值[J]. 临床与病理杂志, 2018, 38(8):1632-1639. doi:10.3978/j.issn.2095-6959.2018.08.007.Zhu CB, Liao GQ, Zhao DM. Prognostic value of Ki-67 index in gastrointestinal stromal tumor[J]. International Journal of Pathology and Clinical Medicine, 2018, 38(8):1632-1639. doi:10.3978/j.issn.2095-6959.2018.08.007.
[6]Shinomura Y, Kinoshita K, Tsutsui S, et al. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors[J]. J. Gastroenterol, 2005, 40(8):775-780. doi: 10.1007/s00535-005-1674-0.
[7]Cameron S, Beham A, Schildhaus HU. Current Standard and Future Perspectives in the Treatment of Gastrointestinal Stromal Tumors[J]. Digestion, 2017, 95(4):262-268. doi: 10.1159/000455835.
[8]熊建明, 黄建华, 李定军, 等. 胃肠道间质瘤临床诊治-附46例临床分析[J]. 现代生物医学进展, 2010, 10(6):1135-1137.Xiong JM, Huang JH, Li DJ, et al. Diagnosis and Treatment of Gastrointestinal Stromal Tumor: Analysis of 46 Cases[J]. Progress in Modern Biomedicine, 2010, 10(6):1135-1137.
[9]Li J, Gong JF, Wu AW, et al. Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor[J]. Eur J Surg Oncol, 2011, 37(4):319-324. doi: 10.1016/j.ejso.2011.01.005.
[10]闫顺笠, 晏仲舒, 廖国庆, 等. 十二指肠间质瘤46例临床诊治分析[J]. 中国普通外科杂志, 2013, 22(10):1324-1328. doi:10.7659/j.issn.1005-6947.2013.10.018.Yan SL, Yan ZS, Liao GQ, et al. Duodenal gastrointestinal stromal tumors:diagnosis and treatment of 46 cases[J]. Chinese Journal of General Surgery, 2013, 22(10):1324-1328. doi:10.7659/j.issn.1005-6947.2013.10.018.
[11]García B, Ibarra J, Sola A, et al. Gastrointestinal stromal tumors. Analysis of 40 cases[J]. Medicina (B Aires), 2017, 77(5):370-372.
[12]Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO[J]. Ann. Oncol, 2005, 16(4):566-578. doi: 10.1093/annonc/mdi127.
[13]中国医师协会外科医师分会胃肠道间质瘤诊疗专业委员会, 中华医学会外科学分会胃肠外科学组. 胃肠间质瘤规范化外科治疗中国专家共识(2018版)[J]. 中国实用外科杂志, 2018, 38(9):965-973. doi: 10.19538/j.cjps.issn.1005-2208.2018.09.01.Committee of Diagnosis and Treatment of Gastrointestinal Stromal Tumor, Chinese College of Surgeons, Gastrointestinal Surgery Group of Surgery Branch, Chinese Medical Association. Chinese expert consensus on standard surgical treatment of gastrointestinal stromal tumor (2018 edition)[J]. Chinese Journal of Practical Surgery, 2018, 38(9):965-973. doi: 10.19538/j.cjps.issn.1005-2208.2018.09.01.
[14]Valsangkar N, Sehdev A, Misra S, et al. Current management of gastrointestinal stromal tumors: Surgery, current biomarkers, mutations, and therapy[J]. Surgery, 2015, 158(5):1149-1164. doi: 10.1016/j.surg.2015.06.027.
[15]Woodall CE 3rd, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system[J]. Arch Surg, 2009, 144(7):670-678. doi: 10.1001/archsurg.2009.108.
[16]Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour[J]. Br J Surg, 2010, 97(12):1854-1859. doi: 10.1002/bjs.7222.
[17]Blesius A, Cassier PA, Bertucci F, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial[J]. BMC Cancer, 2011, 11:72. doi: 10.1186/1471-2407-11-72.
[18]Wang C, Zheng B, Chen Y, et al. Imatinib as preoperative therapy in Chinese patients with recurrent or metastatic GISTs[J]. Chin J Cancer Res, 2013, 25(1):63-70. doi: 10.3978/j.issn.1000-9604.2012.12.01.
[19]Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665[J]. J Surg Oncol, 2009, 99(1):42-47. doi: 10.1002/jso.21160.
[20]Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial[J]. Lancet, 2009, 373(9669):1097-1104. doi: 10.1016/S0140-6736(09)60500-6.
[21]Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial[J]. JAMA, 2012, 307(12):1265-1272. doi: 10.1001/jama.2012.347.
[22]中国临床肿瘤学会胃肠间质瘤专家委员会. 中国胃肠间质瘤诊断治疗共识(2017年版)[J]. 肿瘤综合治疗电子杂志, 2018, 4(1):31-43.Committee of Gastrointestinal Stromal Tumor, Chinese Society of Clinical Oncology. Chinese consensus on diagnosis and treatment of gastrointestinal stromal tumor (2017 edition)[J]. Journal of Esophageal Surgery: Electronic Version, 2018, 4(1):31-43.
[23]中国研究型医院学会消化道肿瘤专业委员会, 中国医师协会外科医师分会多学科综合治疗专业委员会. 胃肠间质瘤多学科综合治疗协作组诊疗模式专家共识[J]. 中国实用外科杂志, 2017, 37(1):39-41. doi: 10.19538/j.cjps.issn1005-2208.2017.01.13.Specialized Committee of Gastrointestinal Tumors of Chinese Research Hospital Association, Specialized Committee of Multidisciplinary Treatment of Chinese Research Hospital Association. Expert consensus on mode of multidisciplinary cooperative group for gastrointestinal stromal tumor[J]. Chinese Journal of Practical Surgery, 2017, 37(1):39-41. doi: 10.19538/j.cjps.issn1005-2208.2017.01.13.
[24]刘安文, 廖国庆, 郑凯, 等. 50例胃肠道间质瘤患者c-kit及PDGFR-a基因检测及突变分析[J]. 中国现代医生, 2013, 51(23):54-56.Liu AW, Liao GQ, Zheng K, et al. Detection of the c-kit and PDGFRα gene mutation and mutation analysis[J]. China Modern Doctor, 2013, 51(23):54-56.
[25]王万川, 廖国庆. 基因突变与胃肠道间质瘤研究进展[J]. 中国普通外科杂志, 2007, 16(9):892-894. doi:10.3969/j.issn. 1005-6947.2007.09.018.Wang WC, Liao GQ. Genetic mutation of gastrointestinal stromal tumors[J]. Chinese Journal of General Surgery, 2007, 16(9):892-894. doi:10.3969/j.issn.1005-6947.2007.09.018.
[26]糜睿, 丁杰, 董奇, 等. 胃肠道间质瘤的基因研究进展[J]. 中国普通外科杂志, 2018, 27(10):1341-1347. doi:10.7659/j.issn.1005-6947.2018.10.018.Mi R, Ding J, Dong Q, et al. Research progress of genes associated with gastrointestinal stromal tumors[J]. Chinese Journal of General Surgery, 2018, 27(10):1341-1347. doi:10.7659/j.issn.1005-6947.2018.10.018.
[27]Lin JX, Chen QF, Zheng CH, et al. Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with highrisk gastrointestinal stromal tumor? A study based on long-term follow-up[J]. J Cancer Res Clin Oncol, 2017, 143(4):727-734. doi: 10.1007/s00432-016-2334-x.
