原发性肝癌(以下简称肝癌)是全世界发生率及病死率最高的恶性肿瘤之一,随着外科技术的发展,越来越多的肝癌患者受益于根治性手术切除,但术后5年复发率仍高达70%[1];术后预防性肝动脉化疗栓塞(transarterial chemoembolization,TACE)是肝癌根治性切除术后常用的预防复发手段之一,TACE能提早发现微病灶、微转移,杀死残留癌组织,提早治疗,有效防止复发,提高生存率[2-4];肿瘤增殖细胞的比例是其生物侵蚀性的重要标志,核抗原Ki-67免疫组化检测是评估细胞增殖最常用的方法[5];目前许多研究已报道Ki-67的表达与肝癌预后密切相关[6-7];而Ki-67评分与肝癌根治性切除术后行预防性TACE患者预后关系未曾被报道。因此,本文采用免疫组化法对我院150例肝癌根治性切除术后行预防性TACE患者的肝癌组织进行Ki-67检测,探讨Ki-67评分与预后的关系。
1 资料与方法
1.1 病例资料
采用回顾性队列研究,收集150例肝癌根治性切除术后行预防性TACE患者的临床病理资料,其中男130例,女20例,中位年龄55(26~76)岁。Ki-67阳性细胞百分数波动于2%~97%,平均(20.42±1.24)%,中位数为20%;根据Ki-67
阳性细胞百分数的中位数进行分组:Ki-67评分≤20%为低表达组,Ki-67评分>20%为高表达组,低表达组44例,高表达组106例。纳入标准:⑴ 术后病理确诊为肝细胞性肝癌;⑵ 术前肝功能Child-Pugh分级A级;⑶ 手术为根治性手术;⑷ 术后只接受1次TACE;⑸ 术前未接受其他抗肿瘤治疗(如射频、TACE、免疫治疗等);⑹ 没有远处转移或手术禁忌。排除标准:⑴ 术前发现远处转移;⑵ 合并其他原发性恶性肿瘤;⑶ 合并影像学及肉眼可见的胆管或血管癌栓;⑷ 术前接受其他非手术治疗;⑸ 临床病理资料不全。复发标准:增强CT、增强MRI及超声造影中两项检查发现肝癌典型“快进快出”特征确诊。本研究通过医院伦理委员会审批,批号为(No.2018-042-01)。
1.2 方法
1.2.1 肝癌根治性切除 术中判断标准:⑴ 肝静脉、门静脉、胆管以及下腔静脉未见肉眼癌栓;⑵ 无邻近脏器侵犯,无肝门淋巴结或远处转移;⑶ 肝脏切缘距肿瘤边界>1 cm;如切缘<1 cm,但切除肝断面组织学检查无肿瘤细胞残留,即切缘阴性。术后判断标准:⑴ 术后2个月行超声、CT、MRI(必须有其中两项)检查未发现肿瘤病灶;⑵ 如术前AFP升高,则要求术后2个月AFP定量测定,其水平在正常范围[8]。
1.2.2 解剖性肝切除与非解剖性肝切除 解剖性肝切除标准:进腹后行术中B超定位,确定拟切除的肝实质范围。在对第一肝门进行解剖时,根据预切除肝脏的范围,解剖、离断预切除肝脏的肝动脉、门静脉。若行肝右前、右后叶、左内叶、左外叶、左半肝、右半肝和右三叶切除时应在第一肝门处离断供应上述肝实质的门静脉及肝动脉分支。肝实质离断用钳夹法。常规在切肝时与Glisson鞘一起离断肝管,而不在肝门处游离。对肝断面一般不缝合处理。非解剖性肝切除标准:根据预切除肝脏的部位和大小相应的肝血管阻断方法,本组所有患者均采用Pringle入肝血流阻断法。在距离肿瘤边界1~2 cm处电刀电凝标记出预切除线。钳夹法离断肝实质切除肿瘤[9]。
1.2.3 预防性TACE 采用Seldinger法,即经皮穿刺右股动脉,先超选至肝固有动脉,注入化疗药物 5-FU 750 mg 及奥沙利铂 150 mg,微导管超选至肿瘤供血分支,注入碘油与表柔比星30 mg的混合乳液,可加用明胶海绵颗粒,根据病灶大小,血供情况还可以选择载药微球,PVA栓塞颗粒等栓塞材料,栓塞以肿瘤滋养血管不显影为度;TACE治疗时间为根治性切除术后2个月内。
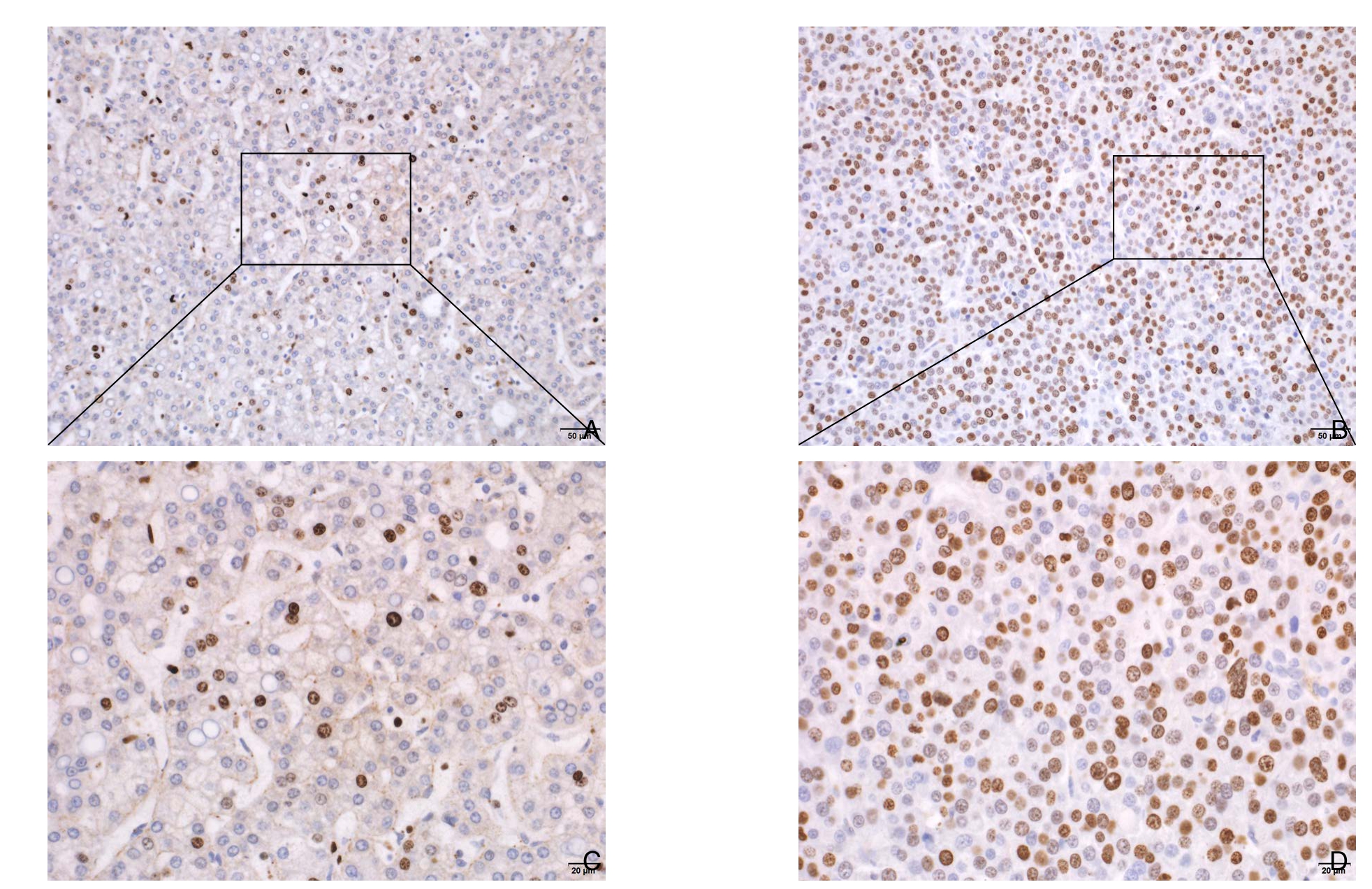
图1 免疫组化检测Ki-67在肝癌中组织中的表达 A:Ki-67低表达(×200);B:Ki-67高表达(×200);C:Ki-67低表达(×400);D:Ki-67高表达(×400)
Figure1 Immunohistochemical staining for Ki-67expression of in liver cancer tissue A:Low Ki-67 expression (×200);B:High Ki-67 expression (×200);C:Low Ki-67 expression (×400);D:High ki-67 expression (×400)
1.2.4 Ki-67检测 所有患者的肿瘤组织样本均采用免疫染色法[10]进行分析。所有肝癌的组织标本均经过10%甲醛固定,常规脱水包埋,切成4 μm厚切片,以小鼠抗人Ki-67单克隆抗体(MIB-1,福州迈新)进行Ki-67免疫染色,低温孵育过夜,漂洗后用3,3'-二氨基联苯胺(DAB,美国 DAKO 公司)显色,最后切片用苏木精复染。结果判定:由两个有经验的病理医师共同阅片,光学显微镜下(×400,Olympus BX53,日本)观察细胞核出现棕黄色颗粒为Ki-67阳性细胞,每张切片选取5个高倍视野随机观察,每个视野计数100个细胞,计算出每个视野的阳性细胞百分数,取其平均值(图1)。
1.2.5 随访 采用住院复查和门诊电话方式进行随访,患者预防性TACE术后第1年每3个月复查1次CT或MRI和血清AFP,1年后每半年复查1次CT或MRI,一旦诊断复发,根据情况行再次手术、射频、TACE、保守治疗,死亡为随访终止时间,随访时间截至2018年1月31日。
1.3 统计学处理
采用χ2检验对分类数据进行比较,用Kaplan-Merier方法估算无病生存期(DFS)和总生存期(OS),采用多变量Cox回归模型的正演方法,进行单因素及多因素分析。P<0.05均被认为具有统计学意义,所有数据均采用SPSS 22.0进行分析。
2 结 果
2.1 临床病理资料分析
分析结果显示,K i-6 7评分高低与肿瘤数目、肿瘤包膜有无、是否合并微血管癌栓有明显关系(均P<0.05),而与性别、年龄、凝血酶原时间(PT)、总胆红素(TBIL)水平、白蛋白(ALB)水平、HBV-DNA载量、AFP水平、是否合并肝硬化、手术方式、肿瘤部位、肿瘤大小、切缘距离、分化程度均无明显关系(均P>0.05)(表1)。
表1 Ki-67表达与临床病理因素的关系[n(%)]
Table1 Relations of Ki-67 expression with the clinicopathologic factors[n (%)]
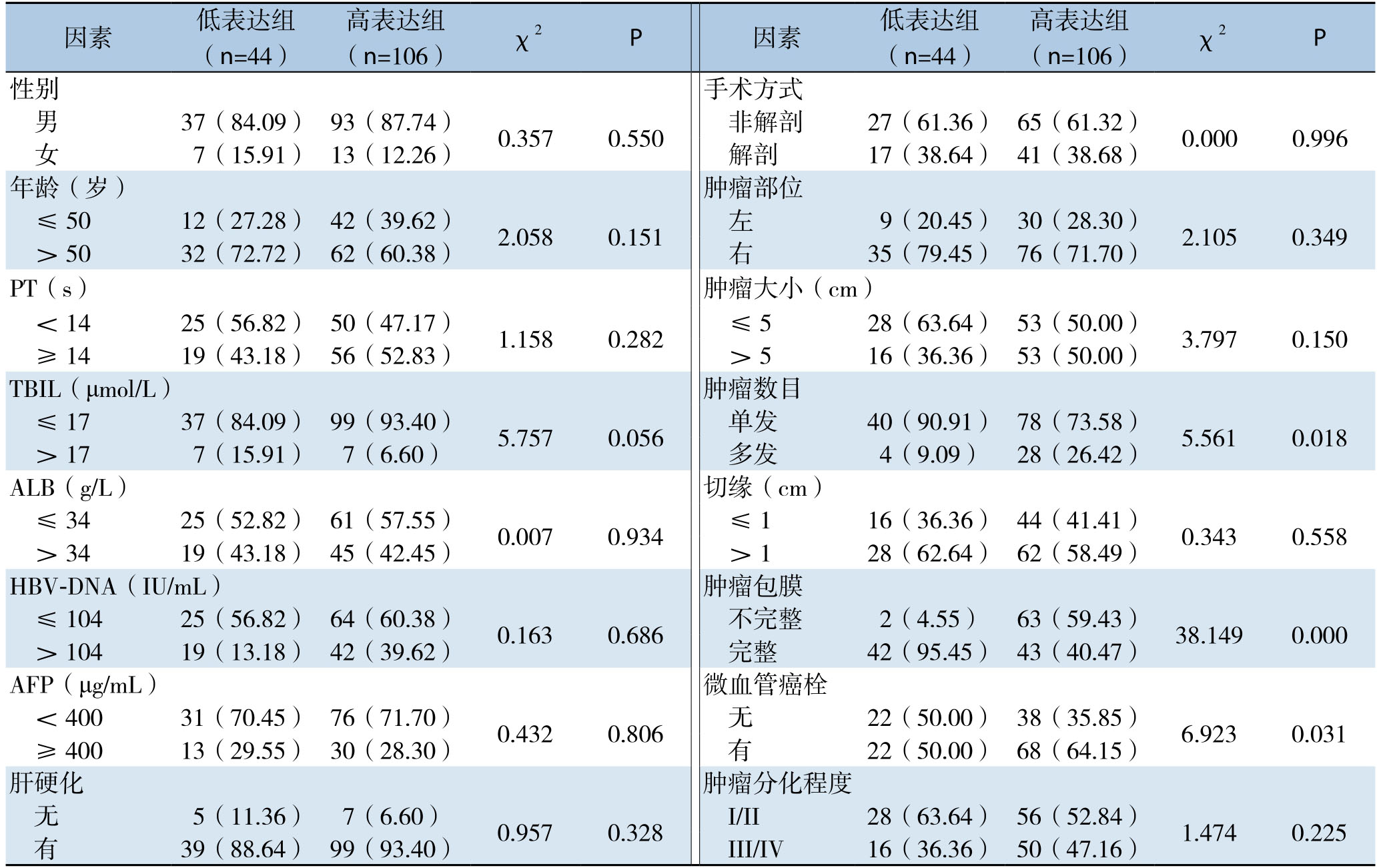
因素 低表达组(n=44) (n=106) χ2 P 因素 低表达组(n=44)高表达组高表达组(n=106) χ2 P 37(84.09) 93(87.74) 0.357 0.550 非解剖 27(61.36) 65(61.32) 0.000 0.996女7(15.91) 13(12.26) 解剖 17(38.64) 41(38.68)年龄(岁) 肿瘤部位≤50 12(27.28) 42(39.62) 2.058 0.151 左 9(20.45) 30(28.30) 2.105 0.349>50 32(72.72) 62(60.38) 右 35(79.45) 76(71.70)PT(s) 肿瘤大小(cm)<14 25(56.82) 50(47.17) 1.158 0.282 ≤5 28(63.64) 53(50.00) 3.797 0.150≥14 19(43.18) 56(52.83) >5 16(36.36) 53(50.00)TBIL(μmol/L) 肿瘤数目≤17 37(84.09) 99(93.40) 5.757 0.056 单发 40(90.91) 78(73.58) 5.561 0.018>17 7(15.91) 7(6.60) 多发 4(9.09) 28(26.42)ALB(g/L) 切缘(cm)≤34 25(52.82) 61(57.55) 0.007 0.934 ≤1 16(36.36) 44(41.41) 0.343 0.558>34 19(43.18) 45(42.45) >1 28(62.64) 62(58.49)HBV-DNA(IU/mL) 肿瘤包膜≤104 25(56.82) 64(60.38) 0.163 0.686 不完整 2(4.55) 63(59.43) 38.149 0.000>104 19(13.18) 42(39.62) 完整 42(95.45) 43(40.47)AFP(μg/mL) 微血管癌栓<400 31(70.45) 76(71.70) 0.432 0.806 无 22(50.00) 38(35.85) 6.923 0.031≥400 13(29.55) 30(28.30) 有 22(50.00) 68(64.15)肝硬化 肿瘤分化程度无5(11.36) 7(6.60) 0.957 0.328 I/II 28(63.64) 56(52.84) 1.474 0.225有39(88.64) 99(93.40) III/IV 16(36.36) 50(47.16)性别 手术方式男
2.2 影响肝癌根治性切除术后行预防性TACE患者DFS及OS的单因素及多因素分析
影响DFS单因素分析:AFP>400 ng/mL、非解剖性肝切除、肿瘤多发、肿瘤大、Ki-67高表达为DFS的影响因素(均P<0.05);影响DFS多因素分析:肿瘤多发、肿瘤大、Ki-67高表达为影响DFS的独立危险因素(均P<0.05)(表2)。影响OS单因素分析:男性、肿瘤多发、肿瘤大、肿瘤包膜不完整、合并微血管癌栓、Ki-67高表达为OS的影响因素影(均P<0.05);影响OS多因素分析:肿瘤多发、肿瘤大、肿瘤包膜不完整、合并微血管癌栓、Ki-67高表达为影响OS的独立危险因素(均P<0.05)(表3)。
表2 原发性肝癌根治性切除术后行预防性TACE患者DSF危险因素分析
Table2 Analysis of the risk factors for DSF in patients with prophylactic TACE after radical hepatectomy for primary liver cancer
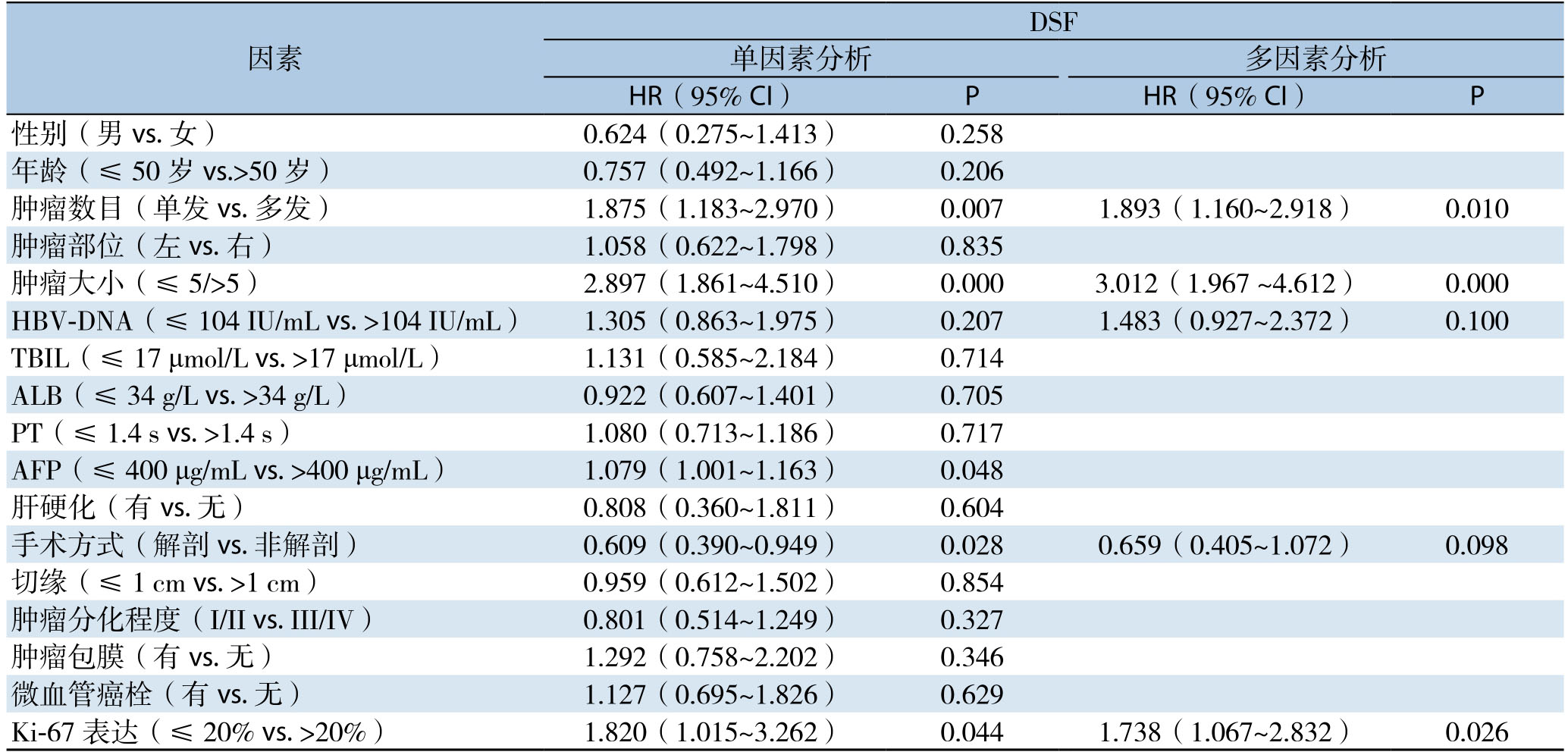
因素DSF单因素分析 多因素分析HR(95% CI) P HR(95% CI) P性别(男vs.女) 0.624(0.275~1.413) 0.258年龄(≤50岁vs.>50岁) 0.757(0.492~1.166) 0.206肿瘤数目(单发vs.多发) 1.875(1.183~2.970) 0.007 1.893(1.160~2.918) 0.010肿瘤部位(左vs.右) 1.058(0.622~1.798) 0.835肿瘤大小(≤ 5/>5) 2.897(1.861~4.510) 0.000 3.012(1.967 ~4.612) 0.000 HBV-DNA(≤ 104 IU/mL vs.>104 IU/mL) 1.305(0.863~1.975) 0.207 1.483(0.927~2.372) 0.100 TBIL(≤ 17 μmol/L vs.>17 μmol/L) 1.131(0.585~2.184) 0.714 ALB(≤ 34 g/L vs.>34 g/L) 0.922(0.607~1.401) 0.705 PT(≤ 1.4 s vs.>1.4 s) 1.080(0.713~1.186) 0.717 AFP(≤ 400 μg/mL vs.>400 μg/mL) 1.079(1.001~1.163) 0.048肝硬化(有vs.无) 0.808(0.360~1.811) 0.604手术方式(解剖vs.非解剖) 0.609(0.390~0.949) 0.028 0.659(0.405~1.072) 0.098切缘(≤ 1 cm vs.>1 cm) 0.959(0.612~1.502) 0.854肿瘤分化程度(I/II vs.III/IV) 0.801(0.514~1.249) 0.327肿瘤包膜(有vs.无) 1.292(0.758~2.202) 0.346微血管癌栓(有vs.无) 1.127(0.695~1.826) 0.629 Ki-67表达(≤20% vs.>20%) 1.820(1.015~3.262) 0.044 1.738(1.067~2.832) 0.026
表3 原发性肝癌根治性切除术后行预防性TACE患者OS危险因素分析
Table3 Analysis of the risk factors for OS in patients with prophylactic TACE after radical hepatectomy for primary liver cancer
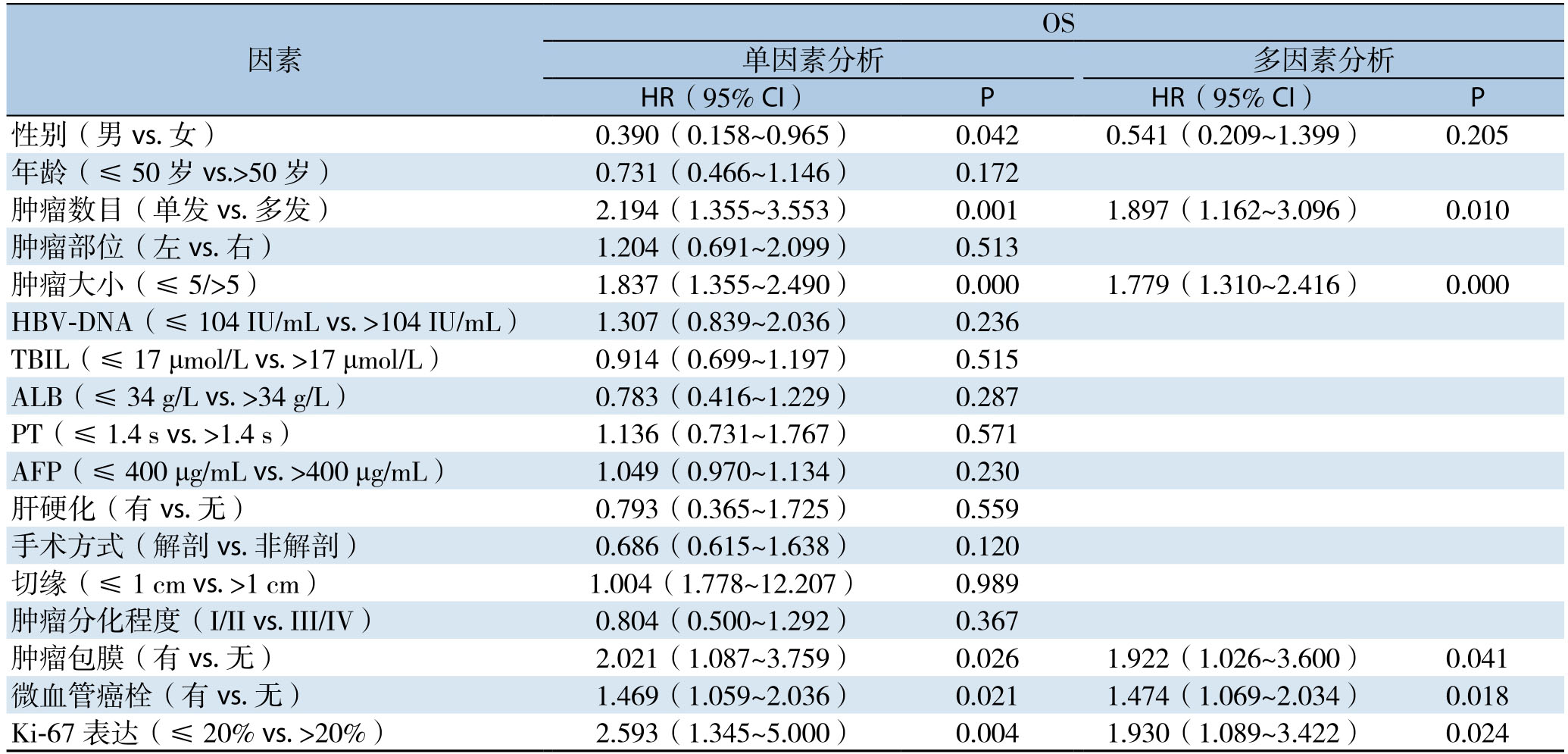
因素OS单因素分析 多因素分析HR(95% CI) P HR(95% CI) P性别(男vs.女) 0.390(0.158~0.965) 0.042 0.541(0.209~1.399) 0.205年龄(≤50岁vs.>50岁) 0.731(0.466~1.146) 0.172肿瘤数目(单发vs.多发) 2.194(1.355~3.553) 0.001 1.897(1.162~3.096) 0.010肿瘤部位(左vs.右) 1.204(0.691~2.099) 0.513肿瘤大小(≤5/>5) 1.837(1.355~2.490) 0.000 1.779(1.310~2.416) 0.000 HBV-DNA(≤ 104 IU/mL vs.>104 IU/mL) 1.307(0.839~2.036) 0.236 TBIL(≤ 17 μmol/L vs.>17 μmol/L) 0.914(0.699~1.197) 0.515 ALB(≤ 34 g/L vs.>34 g/L) 0.783(0.416~1.229) 0.287 PT(≤ 1.4 s vs.>1.4 s) 1.136(0.731~1.767) 0.571 AFP(≤ 400 μg/mL vs.>400 μg/mL) 1.049(0.970~1.134) 0.230肝硬化(有vs.无) 0.793(0.365~1.725) 0.559手术方式(解剖vs.非解剖) 0.686(0.615~1.638) 0.120切缘(≤ 1 cm vs.>1 cm) 1.004(1.778~12.207) 0.989肿瘤分化程度(I/II vs.III/IV) 0.804(0.500~1.292) 0.367肿瘤包膜(有vs.无) 2.021(1.087~3.759) 0.026 1.922(1.026~3.600) 0.041微血管癌栓(有vs.无) 1.469(1.059~2.036) 0.021 1.474(1.069~2.034) 0.018 Ki-67表达(≤20% vs.>20%) 2.593(1.345~5.000) 0.004 1.930(1.089~3.422) 0.024
2.3 Ki-67评分与肝癌根治性切除术后行预防性TACE患者DFS及OS关系
低表达组和高表达组复发率分别为37.7%和57.9%,两组患者无瘤生存期差异有统计学意义(χ2=6.777,P<0.05)。低表达组和高表达组患者生存率分别为75.9%和45.6%,两组比较,差异有统计学意义(χ2=8.447,P<0.05)(图2)。
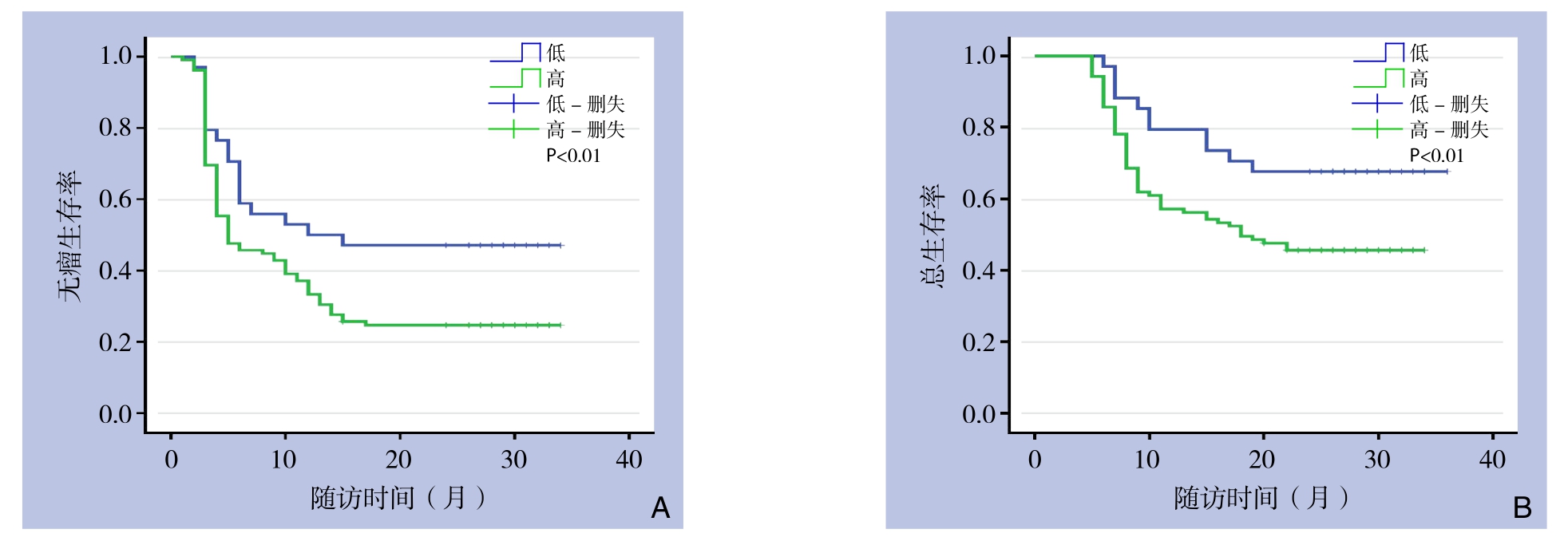
图2 Ki-67高表达组与低表达组患者Kaplan-Meier生存曲线 A:DFS曲线;B:OS曲线
Figure2 Kaplan-Meier survival curves of the patients in high and low Ki-67 expression groups A:DFS curves;B:OS curves
3 讨 论
肝癌根治性切除术后行预防性TACE能提早发现微病灶、微转移,杀死残留癌组织,改善患者预后[11-12],且载药微球与传统碘油栓塞效果无显著差别[13];近年来,许多学者提出仅部分肝癌患者能从预防性TACE中获益,研究[2,14-15]发现按MVI等级分组后合并MVI组更能从预防性TACE治疗中获益;Liao等[16]指出当肝癌直径>5 cm更能从预防性TACE治疗中获益;Hsiao等[17]发现在多发性肝癌的患者中,肝内转移比多中心起源的患者更能从预防性TACE治疗中获益;此外,Huang等[18]结合肿瘤包膜、肿瘤大小、肿瘤数目、肿瘤切缘及MVI设计预防性TACE治疗获益评分系统,得分越高,越能从中获益。本文旨在探讨Ki-67评分与肝癌根治性切除术后行预防性TACE患者预后关系。
肿瘤细胞的增殖状态是反应肿瘤生物学特性的重要参数[19],影响肿瘤术后治疗的有效性;Ki-67是一种传统的增殖标志物,在细胞周期的G1、S和G2期达到高峰,而在G0期缺失,在许多肿瘤中高表达,Ki-67标志物的变化能可靠地反映正常和病变组织的增殖活性,对肿瘤的复发和转移有预测作用;众多研究已报道Ki-67与肿瘤临床病理特征及预后的关系,包括:乳腺癌、垂体腺瘤、喉癌、肾上腺皮质癌、套淋巴瘤和卵巢癌等[20-24]。目前很多研究表明Ki-67与肝癌预后密切相关[25-29],肝癌组织Ki-67高表达常常与组织分化差、肿瘤大、淋巴结转移、血管侵犯及肝外转移相关;有研究表明,Ki-67降低细胞间的黏附力,使肿瘤细胞从肿瘤分离并与细胞外基质黏附,能促进肿瘤的转移[30],本研究同样发现,在Ki-67高低表达组中,肿瘤包膜是否完整、肿瘤数目、是否合并微血管癌栓的差异有统计学意义,与上述研究结果相仿。
以往的研究中[2,15,18]表明,肝癌根治性术后合并高危因素如肿瘤大、MVI、肿瘤多发等建议行TACE术,Ki-67常常与这些肝癌复发高危因素相关[31];然而本研究结果提示,在肝癌根治性切除术后行预防性TACE患者中,Ki-67低表达组无瘤生存期及总生存期均超过Ki-67高表达组,原因可能是预防性TACE仅仅行肝动脉栓塞,而Ki-67高表达组微血管侵犯率高于低表达组(64.15% vs.50%,P<0.05),导致早期复发,影响预后;因此,在评估原发性肝癌根治术后行预防性TACE患者预后时,Ki-67表达可能是一个重要参考指标。
本研究多因素分析结果显示肿瘤多发、肿瘤大、Ki-67高表达是影响肝癌根治性切除术患者预后的独立危险因素,这与以往的报道相一致;研究[32]显示肿瘤的直径<5 cm的肝癌患者根治术后5年生存率为58.2%、10年生存率为38.4%,而肿瘤直径>5 cm的肝癌患者根治术后5年生存率下降至31.4%、10年生存率降至20.43%;研究[33]显示肿瘤单发的肝癌患者根治术后5年生存率为65.8%,而肿瘤多发的肝癌患者根治术后5年生存率下降至45.8%。
本研究仍有如下不足:⑴ 单中心并且是回顾性研究,一个前瞻、多中心、随机的临床试验是需要的;⑵ 本研究未纳入丙型肝炎、酒精性肝炎及脂肪肝相关性肝癌;⑶ 患者复发后不同治疗方式可能影响研究结果,需进一步探究。
综上所述,Ki-67高表达为肝癌根治性切除术后行预防性TACE患者预后不良因素之一。
[1]European Association for the Study of the Liver.EASL Clinical Practice Guidelines:Management of hepatocellular carcinoma[J].J Hepatol,2018,69(1):182-236.doi:10.1016/j.jhep.2018.03.019.
[2]Chen X,Zhang B,Yin X,et al.Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection[J].J Cancer Res Clin Oncol,2013,139(5):773-781.doi:10.1007/s00432-012-1343-7.
[3]Li F,Guo Z,Zhang Y,et al.Postoperative adjuvant arterial chemoembolization improves the survival of hepatitis B virusrelated hepatocellular carcinoma:a retrospective control study[J].Ir J Med Sci,2015,184(4):753-759.doi:10.1007/s11845-014-1164-6.
[4]Kuramoto K,Beppu T,Nitta H,et al.Hepatic Resection Followed by Hepatic Arterial Infusion Chemotherapy for Hepatocellular Carcinoma with Intrahepatic Dissemination[J].Anticancer Res,2018,38(1):525-531.doi:10.21873/anticanres.12254.
[5]Miller I,Min M,Yang C,et al.Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence[J].Cell Rep,2018,24(5):1105-1112.doi:10.1016/j.celrep.2018.06.110.
[6]Yang C,Su H,Liao X,et al.Marker of proliferation Ki-67 expression is associated with transforming growth factor beta 1 and can predict the prognosis of patients with hepatic B virus-related hepatocellular carcinoma[J].Cancer Manag Res,2018,10:679-696.doi:10.2147/CMAR.S162595.
[7]Shi W,Hu J,Zhu S,et al.Expression of MTA2 and Ki-67 in hepatocellular carcinoma and their correlation with prognosis[J].Int J Clin Exp Pathol,2015,8(10):13083-13089.
[8]中华人民共和国卫生和计划生育委员会医政医管局.原发性肝癌诊疗规范(2017年版)[J].中华肝脏病杂志,2017,25(12):886-895.doi:10.3760/cma.j.issn.1007-3418.2017.12.002.
Bureau of Medical Administration,National Health and Family Planning Committee.Diagnosis,management,and treatment of hepatocellular carcinoma(V2017)[J].Chinese Journal of Hepatology,2017,25(12):886-895.doi:10.3760/cma.j.issn.1007-3418.2017.12.002.
[9]池闽辉,曾永毅,刘景丰.解剖性与非解剖性肝切除治疗肝细胞癌的疗效比较研究[J].福建医科大学学报,2012,46(5):327-330.doi:10.3969/j.issn.1672-4194.2012.05.008.
Chi MH,Zeng YY,Liu JF.Comparative Study on Therapeutic Effect between Anatomical Liver Resection and Nonanatomical Liver Resection for Hepatocellular Carcinoma Patients[J].Journal of Fujian Medical University,2012,46(5):327-330.doi:10.3969/j.issn.1672-4194.2012.05.008.
[10]Singla S,LeVea CM,Pokuri VK,et al.Ki67 score as a potential predictor in the selection of liver-directed therapies for metastatic neuroendocrine tumors:a single institutional experience[J].J Gastrointest Oncol,2016,7(3):441-448.doi:10.21037/jgo.2016.02.02.
[11]Wen J,Shen WL,Yang SH.Adjuvant hepatic chemotherapy after resection of solitary hepatocellular carcinoma associated with hepatitis B virus cirrhosis[J].Hepatobiliary Pancreat Dis Int,2006,5(2):224-227.
[12]Lau WY,Lai EC,Leung TW,et al.Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma:a prospective randomized trial-update on 5-year and 10-year survival[J].Ann Surg,2008,247(1):43-48.doi:10.1097/SLA.0b013e3181571047.
[13]Duan F,Wang EQ,Lam MG,et al.Superselective Chemoembolization of HCC:Comparison of Short-term Safety and Efficacy between Drug-eluting LC Beads,QuadraSpheres,and Conventional Ethiodized Oil Emulsion[J].Radiology,2016,278(2):612-621.doi:10.1148/radiol.2015141417.
[14]Nitta H,Beppu T,Imai K,et al.Adjuvant hepatic arterial infusion chemotherapy after hepatic resection of hepatocellular carcinoma with macroscopic vascular invasion[J].World J Surg,2013,37(5):1034-1042.doi:10.1007/s00268-013-1957-1.
[15]Sun JJ,Wang K,Zhang CZ,et al.Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion[J].Ann Surg Oncol,2016,23(4):1344-1351.doi:10.1245/s10434-015-5008-z.
[16]Liao M,Zhu Z,Wang H,et al.Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma:a meta-analysis[J].Scand J Gastroenterol,2017,52(6/7):624-634.doi:10.1080/00365521.2017.1292365.
[17]Hsiao JH,Tsai CC,Liang TJ,et al.Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment[J].Int J Surg,2017,45:35-41.doi:10.1016/j.ijsu.2017.07.071.
[18]Huang LF,Xing X,Wu D,et al.A novel scoring system predicts adjuvant chemolipiodolization benefit for hepatocellular carcinoma patients after hepatectomy[J].Oncotarget,2016,7(18):25493-25506.doi:10.18632/oncotarget.8333.
[19]Scholzen T,Gerdes J.The Ki-67 protein:from the known and the unknown[J].J Cell Physiol,2000,182(3):311-322.doi:10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9.
[20]Chiloiro S,Bianchi A,Doglietto F,et al.Radically resected pituitary adenomas:prognostic role of Ki 67 labeling index in a monocentric retrospective series and literature review[J].Pituitary,2014,17(3):267-276.doi:10.1007/s11102-013-0500-6.
[21]Mahmoud AM,Macias V,Al-Alem U,et al.Ki67 is an independent prognostic marker in breast cancer even after accounting for molecular subtype[J].Cancer Res,2014,74(19 Supplement):5569.
[22]Battista MJ,Mantai N,Sicking I,et al.Ki-67 as an independent prognostic factor in an unselected cohort of patients with ovarian cancer:results of an explorative,retrospective study[J].Oncol Rep,2014,31(5):2213-2219.doi:10.3892/or.2014.3079.
[23]Beuschlein F,Weigel J,Saeger W,et al.Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection[J].J Clin Endocrinol Metab,2015,100(3):841-849.doi:10.1210/jc.2014-3182.
[24]Hoster E,Rosenwald A,Berger F,et al.Prognostic Value of Ki-67 Index,Cytology,and Growth Pattern in Mantle-Cell Lymphoma:Results From Randomized Trials of the European Mantle Cell Lymphoma Network[J].J Clin Oncol,2016,34(12):1386-1394.doi:10.1200/JCO.2015.63.8387.
[25]D'Errico A,Grigioni WF,Fiorentino M,et al.Overexpression of p53 protein and Ki67 proliferative index in hepatocellular carcinoma:an immunohistochemical study on 109 Italian patients[J].Pathol Int,1994,44(9):682-687.
[26]Schmilovitz-Weiss H,Tobar A,Halpern M,et al.Tissue expression of squamous cellular carcinoma antigen and Ki67 in hepatocellular carcinoma-correlation with prognosis:A historical prospective study[J].Diagn Pathol,2011,6:121.doi:10.1186/1746-1596-6-121.
[27]Chen WJ,He DS,Tang RX,et al.Ki-67 is a Valuable Prognostic Factor in Gliomas:Evidence from a Systematic Review and Metaanalysis[J].Asian Pac J Cancer Prev,2015,16(2):411-420.doi:10.7314/apjcp.2015.16.2.411.
[28]Bai K,Cao Y,Huang Q,et al.Prognostic Value of Ki67 Expression for Patients with Surgically Resected Hepatocellular Carcinoma:Perspectives from a High Incidence Area[J].Clin Lab,2017,63(2):355-364.doi:10.7754/Clin.Lab.2016.160638.
[29]Cao Y,Ke R,Wang S,et al.DNA topoisomerase IIα and Ki67 are prognostic factors in patients with hepatocellular carcinoma[J].Oncol Lett,2017,13(6):4109-4116.doi:10.3892/ol.2017.5999.
[30]Chung JH,Lee WW,Park SY,et al.FDG uptake and glucose transporter type 1 expression in lymph nodes of non-small cell lung cancer[J].Eur J Surg Oncol,2006,32(9):989-995.doi:10.1016/j.ejso.2006.05.017.
[31]Li HH,Qi LN,Ma L,et al.Effect of KI-67 positive cellular index on prognosis after hepatectomy in Barcelona Clinic Liver Cancer stage A and B hepatocellular carcinoma with microvascular invasion[J].Onco Targets Ther,2018,11:4747-4754.doi:10.2147/OTT.S165244.
[32]樊嘉,周俭,吴志全,等.原发性肝癌的外科治疗:20年7566例的临床经验[J].中华消化外科杂志,2009,8(2):99-102.doi:10.3760/cma.j.issn.1673-9752.2009.02.009.
Fan J,Zhou J,Wu ZQ,et al.Surgical treatment of primary hepatoeellular carcinoma:a 20-year clinical experience in 7566 patients[J].Chinese Journal of Digestive Surgery,2009,8(2):99-102.doi:10.3760/cma.j.issn.1673-9752.2009.02.009.
[33]朱倩,乔国梁,晏建军,等.乙肝肝硬化相关早期肝癌切除术预后[J].中华肝胆外科杂志,2014,20(4):258-264.doi:10.3760/cma.j.issn.1007-8118.2014.04.006.
Zhu Q,Qiao GL,Yan JJ,et al.Prognosis after resection of early hepatocellular carcinoma in HBV-related cirrhotic patients[J].Chinese Journal of Hepatobiliary Surgery,2014,20(4):258-264.doi:10.3760/cma.j.issn.1007-8118.2014.04.006.
