迷走右锁骨下动脉(aberrant right subclavian artery,ARSA)是一种常见的主动脉弓部发育畸形,发病率为0.5%~1%[1]。当Stanford B型主动脉夹层(TBAD)合并ARSA,由于TBAD原发破口常位于降主动脉峡部,临近ARSA的主动脉开口处,解剖学情况复杂。既往多采用开放手术治疗,手术难度较大。随着胸主动脉覆膜支架腔内技术(thoracic endovascular aortic repair,TEVAR)迅速发展,其已经成为TBAD的首选治疗方法。如何采用TEVAR技术治疗合并ARSA的TBAD是目前临床难点问题。
本研究回顾分析中南大学湘雅二医院血管外科收治的16例采用TEVAR技术治疗的TBAD合并ARSA患者的临床资料,探讨该技术治疗的有效性、安全性和预后。
1 资料与方法
1.1 临床资料
2012年1月—2019年12月共收治急性TBAD合并ARSA患者16例,全部采用TEVAR技术治疗。男14例,女2例;平均年龄为(56.1±11.3)岁,患有5年以上高血压病史14例,脑梗2例,心脏疾患2例,糖尿病1例,慢性阻塞性肺疾病2例,5年以上吸烟史者11例,伴有高血脂症者11例。
所有患者在症状出现后1~11d,平均(4.1±1.8)d入住我院。所有患者入院后行急诊主动脉CTA检查(图1),明确主动脉疾病诊断、解剖学形态特征、和分支动脉受累及情况。根据Criado[2]主动脉弓分区,13例位于Z3区,3例位于Z4区;左椎动脉优势血管14例,右椎动脉优势血管1例,椎动脉均势血管1例;TBAD累及腹腔干9例,累及肠系膜上动脉9例,累及左/右肾动脉 9例,累及髂动脉7例,累及股动脉1例。
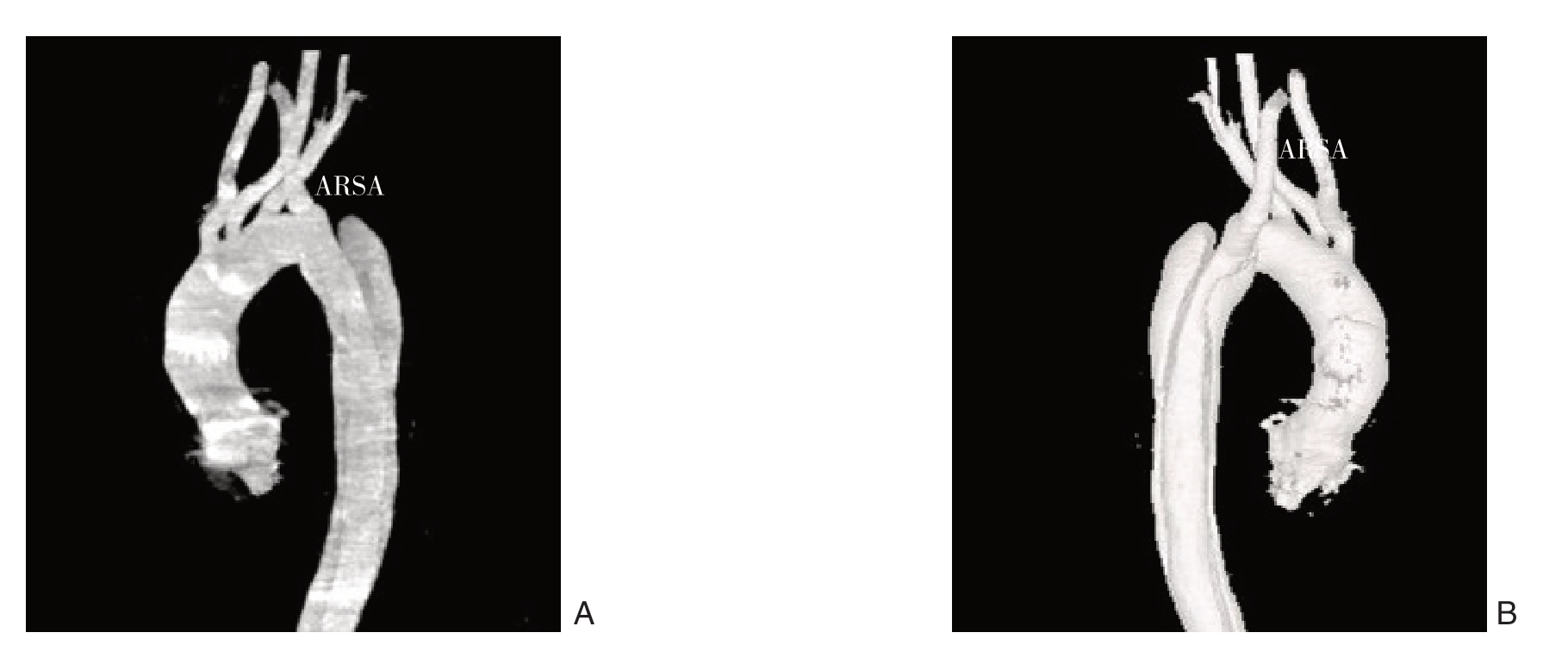
图1 TBAD合并ARSA术前CTA三维重建 A:前视图;B:后视图
Figure1 Three-dimensional reconstruction of preoperative CT angiography of a patient with TBAD and combined ARSA A:Front view;B:Rear view
1.2 手术方法
因为TBAD原发破口位于左锁骨下动脉(left subclavian artery,LSA)起始部附近或累及ARSA的主动脉开口,近端锚定区不足,主动脉支架需向近端前移覆盖LSA和(或)ARSA来取得足够长的锚定区。但同时覆盖LSA和ARSA,有造成锁骨下动脉窃血或上肢缺血,脊髓缺血和脑卒中发生的可能。因此,必须至少保证椎动脉优势一侧锁骨下动脉的血流供应。为制定合理的手术方案,根据TBAD原发破口与LSA、ARSA之间的距离和相对位置,本研究将TBAD合并ARSA分为以下3种情况:⑴ TBAD原发破口位于ARSA开口以远,且距离>15 mm,行TEVAR封堵夹层破口。⑵ TBAD原发破口位于ARSA开口以远,但距离<15 mm,则根据优势椎动脉决定手术方案:若为左侧椎动脉优势,则行TEVAR封堵夹层破口及ARSA开口;若为右侧椎动脉优势或双侧椎动脉均势,则在行TEVAR的同时重建ARSA。⑶ TBAD原发破口位于LSA开口以远,且距离<15 mm,ARSA受累,根据优势椎动脉决定手术方案:若为左侧椎动脉优势,则重建LSA;若为右椎动脉优势,则重建ARSA;若为双椎均势,则需重建双侧锁骨下动脉。对于需要重建锁骨下动脉的患者,通常选择TEVAR结合“烟囱”技术重建LSA;“烟囱”或“潜望镜”技术重建ARSA;对于夹层破口位于主动脉小弯侧的患者,也可选择开窗技术以保留锁骨下动脉血流。患者予以全麻插管置于仰卧位,于右侧或左侧(根据患者术前CTA决定)腹股沟区和左侧肘部做切口,解剖显露右/左股总动脉和左肱动脉。在直视下Seldinger技术穿刺股动脉和左肱动脉,置入合适的导管鞘。X线透视下将超滑导丝及黄金标记猪尾导管经股动脉置入至升主动脉,行主动脉造影。根据TBAD破口与LSA和ARSA的位置关系,双侧椎动脉的直径与通畅情况,结合术前CTA结果最终确定手术方案。测量拟锚定区各血管直径,选取与之相适应的主动脉覆膜支架。根据LSA直径大小置入覆膜烟囱支架(通常直径大小为6 mm或8 mm)于LSA,将烟囱支架近端置入主动脉真腔内,远端置于LSA内,从股动脉导入超硬导丝至升主动脉,沿超硬导丝导入选择好的覆膜支架至拟锚定区并准确释放,覆盖原发破口和LSA及ARSA,再释放已置入LSA的覆膜“烟囱”支架,“烟囱”支架的近端需超过主动脉支架近端覆膜部分约10 mm,使用与烟囱支架相同型号的球囊充分扩张LSA“烟囱”支架,扩张压力为8~10 atm(1atm=101.325 kPa)。再次造影确认主动脉夹层修复及各支架通畅情况。撤出各导管导丝,用5-0 Prolene线缝合股动脉和左肱动脉处切口。术毕,患者常规返回病房监测血压、心率等生命体征。
2 结 果
2.1 手术结果及并发症处理
手术时间为50~190min,平均(94.3±41.4)min,16例患者技术成功率100%(表1),其中5例采用单纯TEVAR术覆盖夹层原发破口及ARSA,保留LSA;7例置入主动脉支架和LSA烟囱支架;1例患者采用烟囱技术重建LSA并采用潜望镜技术重建ARSA(图2);1例患者行LSA动脉开窗;2例患者保留了双侧锁骨下动脉。DSA造影示近端原发破口完全修复,主动脉真腔显著扩张,假腔消失,烟囱支架内血流通畅,1例患者出现Ia型内漏,随后予以球囊扩张主动脉支架近端后内漏消失。术后患者均返回病房监测生命体征,术后未发现锁骨下动脉窃血,脊髓缺血,脑卒中等症状,无患者死亡,2例患者出现右上肢乏力症状,予以保守治疗3 d后恢复正常,所有患者均在术后2周内出院。重建LSA和(或)ARSA患者术后口服拜阿司匹林100 mg/d,氯吡格雷75 mg/d,3个月后根据复查CTA显示的支架通畅情况、内漏及假腔血栓化情况决定是否停服。
2.2 随访
随访时间3~66个月,平均(33.2±19.8)个月,所有患者均正常生活,术后2周与3、6、12个月,之后每年1次进行随访,复查CTA。术后2周CTA示烟囱支架和主动脉支架均通畅,真腔扩大,假腔缩小,活动性血流消失(图3)。比较术前和末次随访的主动脉,降主动脉最大直径从术前(36.9±10.1)mm降至(34.0±9.6)mm,假腔与真腔之比从1.04±0.66降至0.20±0.29。长期随访期间,未出现右上肢缺血、脊髓缺血、脑卒中、锁骨下动脉窃血症状,支架移位、内漏和其他支架相关并发症,所有烟囱支架均保持通畅。
表1 手术结果及术中支架使用情况
Table1 Surgical results and stent graft information
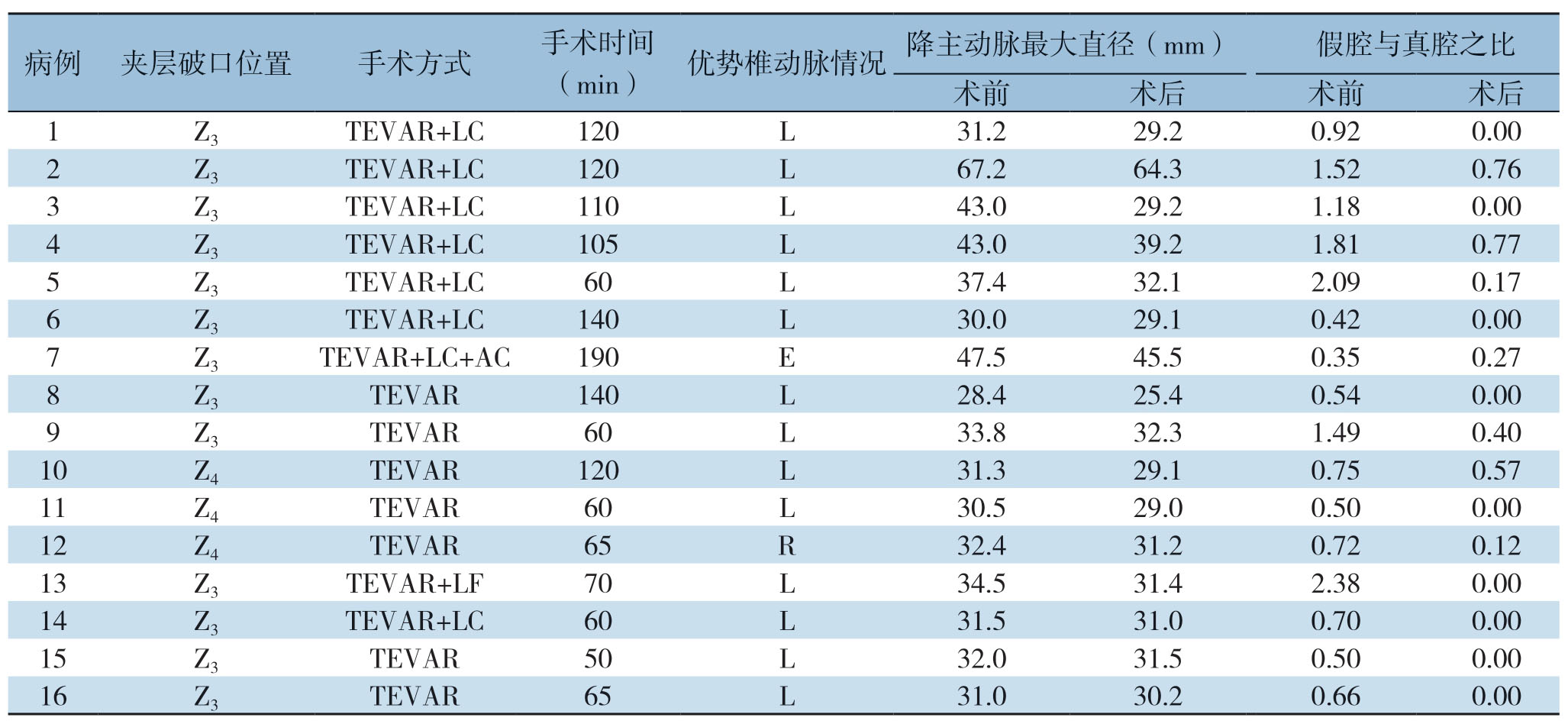
注:LC为左锁骨下动脉烟囱;AC为迷走右锁骨下动脉烟囱;LF为左锁骨下动脉开窗
Note:LC standing for left subclavian artery chimney;AC standing for aberrant subclavian right artery chimney;LF standing for left subclavian artery fenestration
病例 夹层破口位置 手术方式 手术时间(min)优势椎动脉情况 降主动脉最大直径(mm)假腔与真腔之比术前 术后 术前 术后1 Z3 TEVAR+LC 120 L 31.2 29.2 0.92 0.002 Z3 TEVAR+LC 120 L 67.2 64.3 1.52 0.76 3 Z3 TEVAR+LC 110 L 43.0 29.2 1.18 0.004 Z3 TEVAR+LC 105 L 43.0 39.2 1.81 0.77 5 Z3 TEVAR+LC 60 L 37.4 32.12.09 0.17 6 Z3 TEVAR+LC 140 L 30.029.1 0.42 0.007 Z3 TEVAR+LC+AC 190 E 47.5 45.5 0.35 0.27 8 Z3 TEVAR 140 L 28.4 25.4 0.54 0.009 Z3 TEVAR 60 L 33.8 32.3 1.49 0.40 10 Z4 TEVAR 120 L 31.3 29.1 0.75 0.57 11 Z4 TEVAR 60 L 30.5 29.0 0.50 0.0012 Z4 TEVAR 65 R 32.4 31.2 0.72 0.12 13 Z3 TEVAR+LF 70 L 34.5 31.4 2.38 0.0014 Z3 TEVAR+LC 60 L 31.5 31.0 0.70 0.0015 Z3 TEVAR 50 L 32.0 31.5 0.50 0.0016 Z3 TEVAR 65 L 31.0 30.2 0.66 0.00
表1 手术结果及术中支架使用情况(续)
Table1 Surgical results and stent graft information (continued)
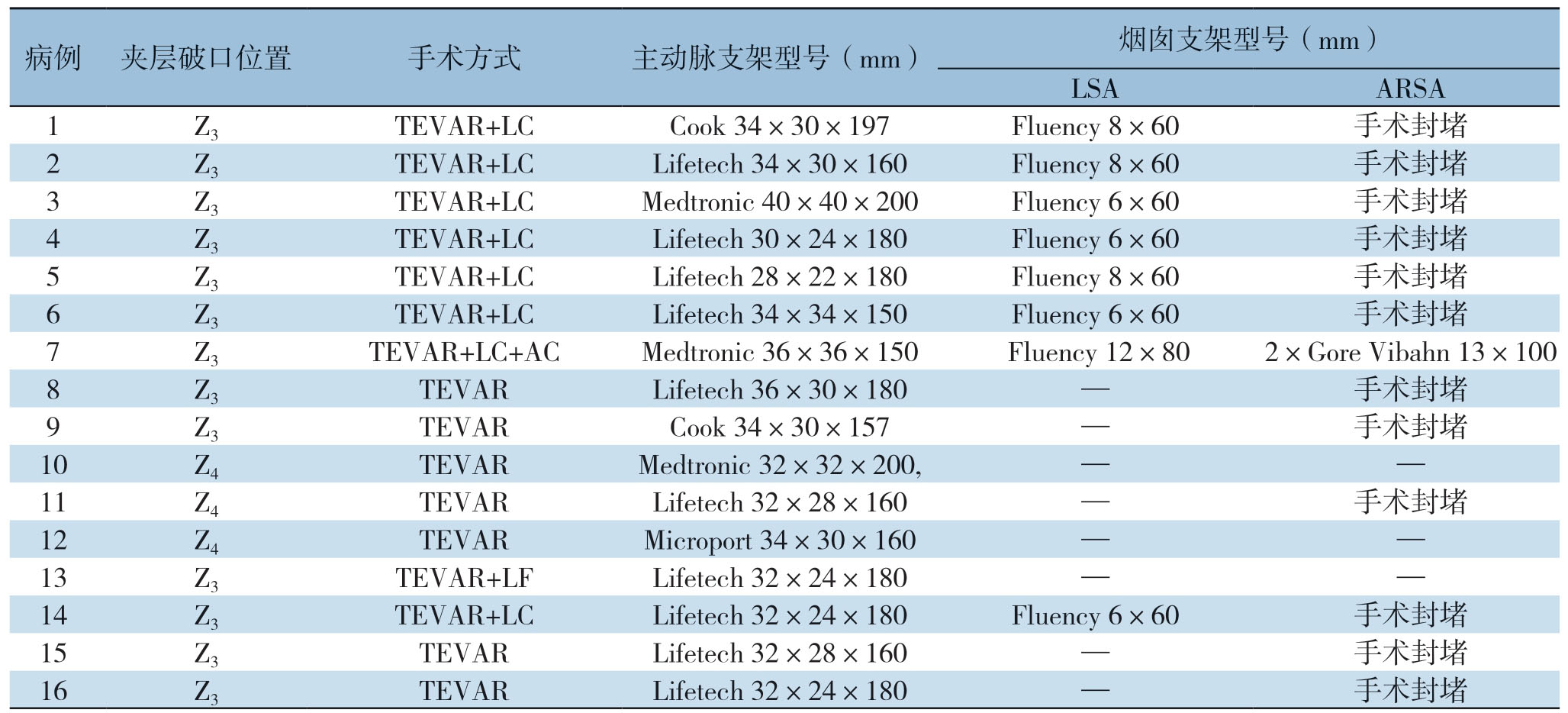
注:LC为左锁骨下动脉烟囱;AC为迷走右锁骨下动脉烟囱;LF为左锁骨下动脉开窗
Note:LC standing for left subclavian artery chimney;AC standing for aberrant subclavian right artery chimney;LF standing for left subclavian artery fenestration
病例 夹层破口位置 手术方式 主动脉支架型号(mm)烟囱支架型号(mm)LSA ARSA 1 Z3 TEVAR+LC Cook 34×30×197 Fluency 8×60 手术封堵2 Z3 TEVAR+LC Lifetech 34×30×160 Fluency 8×60 手术封堵3 Z3 TEVAR+LC Medtronic 40×40×200 Fluency 6×60 手术封堵4 Z3 TEVAR+LC Lifetech 30×24×180 Fluency 6×60 手术封堵5 Z3 TEVAR+LC Lifetech 28×22×180 Fluency 8×60 手术封堵6 Z3 TEVAR+LC Lifetech 34×34×150 Fluency 6×60 手术封堵7 Z3 TEVAR+LC+AC Medtronic 36×36×150 Fluency 12×80 2×Gore Vibahn 13×100 8 Z3 TEVAR Lifetech 36×30×180 — 手术封堵9 Z3 TEVAR Cook 34×30×157 — 手术封堵10 Z4 TEVAR Medtronic 32×32×200,— —11 Z4 TEVAR Lifetech 32×28×160 — 手术封堵12 Z4 TEVAR Microport 34×30×160 — —13 Z3 TEVAR+LF Lifetech 32×24×180 — —14 Z3 TEVAR+LC Lifetech 32×24×180 Fluency 6×60 手术封堵15 Z3 TEVAR Lifetech 32×28×160 — 手术封堵16 Z3 TEVAR Lifetech 32×24×180 — 手术封堵
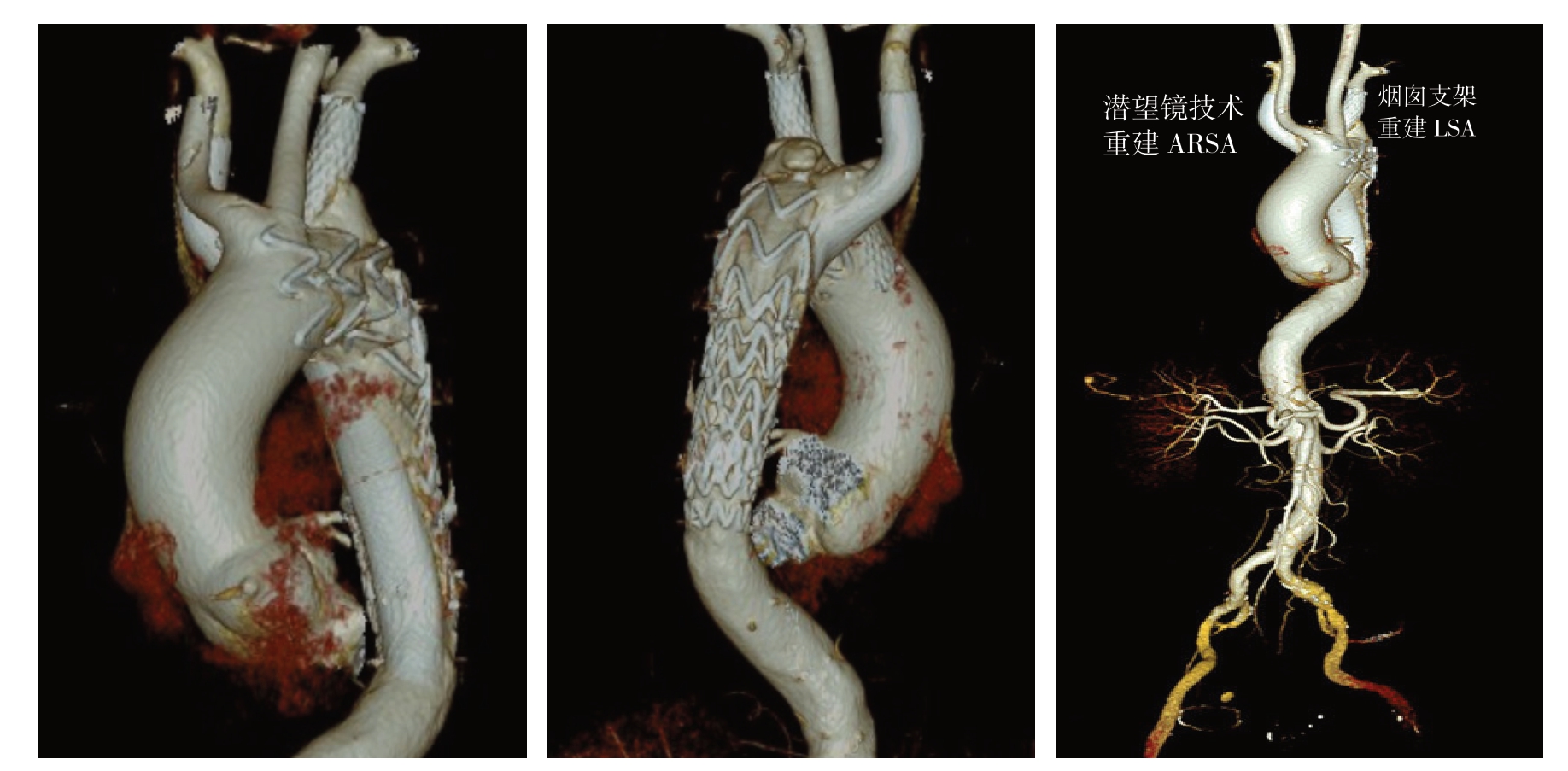
图2 使用烟囱技术重建LSA,潜望镜技术重建ARSA患者的术后CTA三维重建图像(TBAD 被完全封堵,弓部分支动脉通过重建,均保持通畅)
Figure2 Three-dimensional reconstruction of postoperative CT angiography of a patient undergoing TEVAR plus chimney and periscope technique to reconstruct LSA and ARSA (complete coverage of the TBAD and patent reconstructed branch arteries of the aortic arch)
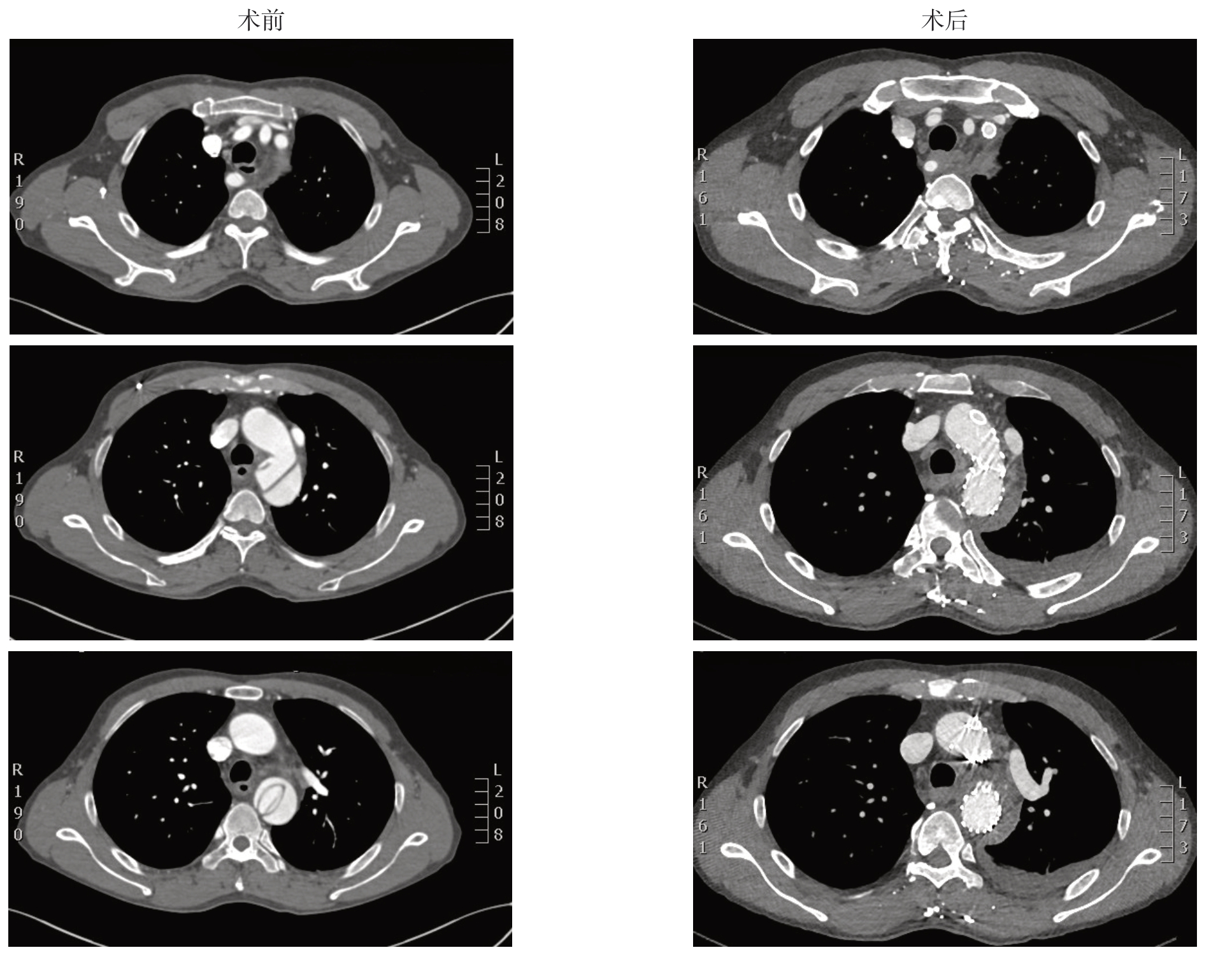
图3 使用TEVAR 联合烟囱技术重建LSA患者的CTA 图像(LSA 内烟囱支架通畅,TBAD 原发破口被主动脉支架封堵,真腔扩大而假腔缩小)
Figure3 The CT angiography of a patient undergoing TEVAR plus chimney technique to reconstruct LSA (patent LSA chimney stent,covarage of the primary tear of aortic dissection by aortic stent,and enlargement of the true lumen with shrinkage of the false lumen)
3 讨 论
ARSA于1735年在尸检中首次发现[3]。ARSA不从无名动脉发出,其作为主动脉弓第四分支,直接开口于左锁骨下动脉以远的主动脉,横穿食管和脊柱之间。ARSA多无症状,约5%的患者有吞咽困难,呼吸困难,胸痛,右上肢乏力等临床表现[4]。
TBAD合并ARSA非常罕见,仅有少量病例报道[5-10]。其发生的机制尚未清楚,推测与动脉形态异常导致的血流动力学改变有关。该病目前尚无统一治疗标准,开胸手术,TEVAR手术,杂交手术均有报道。Kieffer等[11]报道了使用开胸手术治疗TBAD合并ARSA的早期病死率为30%。杂交手术联合外科及腔内技术处理主动脉弓部病变,旨在降低传统开放手术的创伤和技术难度,其手术术式多样且差异较大,目前尚无统一的标准,治疗效果有待研究。Cochennec等 [12]采用杂交手术治疗累及主动脉弓部的复杂TBAD病变,30 d病死率为29%,术后24%的患者出现逆撕的A型夹层,12%的患者出现致死性脑卒中;术后II型和III型内漏发生率也较高,部分需要二期手术治疗。
TEVAR手术创伤小,具有良好的安全性和有效性,是目前TBAD首选的治疗方法[13]。对于合并ARSA的TBAD,TEVAR治疗的报道较少;相比开放手术和杂交手术,其创伤小,避免了主动脉吻合、深低温体外循环等高风险的复杂步骤,具有显著优势,尤其对于高龄和存在严重合并症的患者。TEVAR治疗的关键在于:主动脉弓部分支受累情况,双侧椎动脉的形态,同时主动脉和ARSA扭曲度相对较小[14]。对于合并ARSA的TBAD,ARSA开口和夹层破口相对位置的不确定性,主动脉弓部4分支通常不同程度被TBAD累及,是TEVAR治疗TBAD合并ARSA的主要难点。在某些情况下,夹层破口累及LSA或恰好位于LSA开口处而无足够的锚定区,为使TEVAR获得足够的近端锚定区并保证分支动脉血供,通常需要结合“烟囱”、开窗、分支支架等辅助技术;手术时间因此而延长,操作更为复杂而棘手,围手术期病死率、术后内漏的发生、脑卒中等神经系统的并发症也相应增加[15]。本研究中,技术成功率达100%,无围手术期死亡的发生,无截瘫、脑卒中等严重并发症的发生,证实了TEVAR治疗TBAD合并ARSA这一复杂解剖条件的短中期有效性和安全性。
对于椎动脉、基底动脉正常、Willis环完整且非左椎动脉优势的TBAD患者,TEVAR手术封堵LSA一般不会发生缺血、功能障碍等严重并发症[16]。本研究中,ARSA位于LSA远端且夹层原发破口邻近LSA,直接修复破口可能同时封堵两侧锁骨下动脉,容易导致脊髓缺血、脑卒中等严重并发症,因此,应尽可能的保留脊髓和椎动脉的血流灌注而减少截瘫和脑梗发生率。诚然,完全重建双侧锁骨下动脉能最大限度保留血流灌注,但也会导致技术难度增加和手术时间延长,导致其他手术并发症增加。此外,过多的弓上操作也是导致主动脉弓部及颈部动脉斑块脱落,发生围手术期脑梗的原因之一。因此,至少保留椎动脉优势一侧锁骨下动脉的血流灌注可能是目前最佳的选择。研究中所有患者的椎动脉均发自于锁骨下动脉,其中14例为左椎动脉优势,1例为右椎动脉优势,1例为左右均势椎动脉,7例患者LSA置入烟囱支架保留椎动脉血管灌注。术后随访期间未见脊髓缺血、脑缺血等并发症的发生。
目前重建锁骨下动脉[17]的方法有:杂交技术如行血管搭桥或转流术,开窗、烟囱、潜望镜等技术,Ding等[18]报道应用杂交技术治疗的16例患者中出现腋神经损伤和Ia型内漏的发生率分别为12.5%和18.8%。由于手术相对复杂,杂交手术治疗复杂性TBAD可能导致各种手术相关并发症[12,19-20]。Xiang等[21]报道颈总动脉-锁骨下动脉搭桥术(CSB)有较长的ICU住院时间且随访中总病死率达到了14.3%。Saouti等[22]报道了CSB组中总并发症率高达39%,而Piffaretti等 [23]报道CSB组和烟囱技术组的临床效果及中期随访结果无明显差异。面对差别不大的临床效果,患者通常更愿选择创伤较小的治疗方法。“烟囱”技术适用于近端锚定区不足的复杂主动脉病变的处理。已有研究[24-25]表明,“烟囱”支架术后短期通畅率达100%,有效保障了分支血管的血供。潜望镜技术,又称为“逆向烟囱”技术,为分支支架预置于降主动脉内,与主动脉支架平行,其血流为主动脉远端逆流而上而供应分支动脉[26]。对于需重建双侧锁骨下动脉的患者而言,双烟囱技术有增加内漏的风险,烟囱技术结合潜望镜技术重建双侧锁骨下动脉不失为一种合理分配主动脉锚定区空间,减少主动脉支架与分支支架缝隙进而降低内漏发生的方法。且用潜望镜技术于ARSA中置入支架比烟囱顺行支架更符合主动脉弓部的解剖学形态。本研究有1例患者即采用潜望镜技术合并烟囱技术重建的LSA和ARSA。
开窗技术和分支支架技术也是主动脉弓部疾病腔内修复辅助技术[27],但其临床运用仍有其局限性,尤其是分支支架技术,其对主动脉弓部解剖形态和病变部位较为苛刻,且释放步骤难度大,支架制作耗时、且花费更高[28]。开窗技术分为体外开窗和原位开窗两类,对于需急诊手术的患者,或是高危但经济条件有限的患者,可考虑应用主动脉支架人工体外开窗技术[29],即术者在术中根据主动脉病变的解剖学形态,对主动脉覆膜支架进行体外开窗或开槽,再装入导送鞘内。此项技术需要精准的开口定位,适用于病变位于主动脉弓部小弯侧的患者[15]。本研究中1例患者因其破口位于主动脉弓小弯侧故选择LSA开窗术。由此可见,破口的位置,主要是其在径向和轴向距离弓部分支动脉的距离,对选择合适的腔内技术也是非常重要的。但体外开窗技术最大的风险在于可能出现对位不准,开窗部位不能保留分支动脉,从而导致手术失败,同时增加III型内漏的风险。
因为烟囱支架、主动脉支架和血管壁三者之间存在缝隙,烟囱技术可能导致Ia型内漏的发生[30],但实际上,由于该缝隙狭窄,其中血流速度缓慢等原因可使其内血栓形成,缝隙逐渐消失。因此,怎样选择合适的支架使得烟囱支架和主动脉支架之间的缝隙缩小而易于血栓化显得尤为重要。我们的经验是烟囱支架选择材质较硬、横向抗压能力较强者,而主动脉支架选择质软者,可使两者贴合类似包绕状。由于烟囱支架直径较小且受到主动脉和血管壁的机械压力而存在发生闭塞可能[31],因此在术后治疗中须进行抗血小板祛聚治疗以预防烟囱支架内血栓形成造成闭塞[32],本研究随访期间,所有烟囱支架均血流通畅。
TEVAR技术治疗TBAD合并ARSA是一种可行的微创治疗方法,有效性和安全性较好,能够获得稳定的中长期疗效。主动脉弓部分支动脉的重建是其关键问题,多种腔内分支动脉重建技术可以使用。更深入的研究需要大宗病例的前瞻性研究。
[1]Kiernan PD,Dearani J,Byrne WD,et al.Aneurysm of an aberrant right subclavian artery:case report and review of the literature[J].Mayo Clin Proc,1993,68(5):468-474.doi:10.1016/s0025-6196(12)60196-7.
[2]Criado FJ,Clark NS,Barnatan MF.Stent graft repair in the aortic arch and descending thoracic aorta:a 4-year experience[J].J Vasc Surg,2002,36(6):1121-1128.doi:10.1067/mva.2002.129649.
[3]Hunauld PM.Examen de quelques parties d’unsinge[J].Hist Acad Roy Sci,1735,2:516-523.
[4]Yang C,Shu C,Li M,et al.Aberrant subclavian artery pathologies and Kommerell's diverticulum:a review and analysis of published endovascular/hybrid treatment options[J].J Endovasc Ther,2012,19(3):373-382.doi:10.1583/11-3673MR.1.
[5]Cooper DG,Markur S,Walsh SR,et al.Hybrid endovascular repair of an aneurysmal chronic type B dissection in a patient with Marfan syndrome with an aberrant right subclavian artery[J].Vasc Endovasc Surg,2009,43(3):271-276.doi:10.1177/1538574408328662.
[6]Guzman ED,Eagleton MJ.Aortic dissection in the presence of an aberrant right subclavian artery[J].Ann Vasc Surg,2012,26(6):860.doi:10.1016/j.avsg.2012.01.011.
[7]Hamidian-Jahromi A,Carroll JD,Doucet LD,et al.Hybrid repair of ruptured type B aortic dissection extending into an aberrant right subclavian artery in a patient with Turner's syndrome[J].Ann Vasc Surg,2013,27(8):1182.doi:10.1016/j.avsg.2013.04.007.
[8]Kikuchi K,Makuuchi H,Oono M,et al.Surgery for aortic dissection involving an aberrant right subclavian artery[J].Jpn J Thorac Cardiovasc Surg,2005,53(12):632-634.doi:10.1007/BF02665073.
[9]Mosquera VX,Marini M,Rodríguez F,et al.Complicated acute type B aortic dissection with involvement of an aberrant right subclavian artery and rupture of a thoracoabdominal aortic aneurysm,Crawford type I:successful emergency endovascular treatment[J].J Thorac Cardiovasc Surg,2007,134(4):1055-1057.doi:10.1016/j.jtcvs.2007.06.024.
[10]Vos AW,Wisselink W,Rijbroek A,et al.Endovascular repair of a type B aortic dissection with transposition of a coexistent aberrant subclavian (lusorian) artery[J].J Endovasc Ther,2002,9(4):549-553.doi:10.1177/152660280200900427.
[11]Kieffer E,Bahnini A,Koskas F.Aberrant subclavian artery:surgical treatment in thirty-three adult patients[J].J Vasc Surg,1994,19(1):100-109.doi:10.1016/s0741-5214(94)70125-3.
[12]Cochennec F,Tresson P,Cross J,et al.Hybrid repair of aortic arch dissections[J].J Vasc Surg,2013,57(6):1560-1567.doi:10.1016/j.jvs.2012.11.081.
[13]Brunkwall J,Kasprzak P,Verhoeven E,et al.Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling:1 year results of the ADSORB trial[J].Eur J Vasc Endovasc Surg,2014,48(3):285-291.doi:10.1016/j.ejvs.2014.05.012.
[14]Okada K,Sueda T,Orihashi K,et al.Endovascular stent-graft repair for thoracic aortic aneurysm associated with right-sided aortic arch[J].J Thorac Cardiovasc Surg,2001,122(1):185-186.doi:10.1067/mtc.2001.113019.
[15]Shu C,Fan B,Luo M,et al.Endovascular treatment for aortic arch pathologies:chimney,on-the-table fenestration,and in-situ fenestration techniques[J].J Thorac Dis,2020,12(4):1437-1448.doi:10.21037/jtd.2020.03.10.
[16]Liu H,Shu C,Li X,et al.Endovascular aortic repair combined with chimney technique in the treatment of stanford type B aortic dissection involving aortic arch[J].Ann Vasc Surg,2015,29(4):758-763.doi:10.1016/j.avsg.2014.12.004.
[17]刘子豪,郗二平.Stanford B型主动脉夹层腔内修复术中左锁骨下动脉处理的研究进展[J].中国普通外科杂志,2017,26(6):781-788.doi:10.3978/j.issn.1005-6947.2017.06.018.
Liu ZH,Xi EP.Managements of left subclavian artery during endovascular repair of Stanford type B aortic dissection:recent advances[J].Chinese Journal of General Surgery,2017,26(6):781-788.doi:10.3978/j.issn.1005-6947.2017.06.018.
[18]Ding H,Luo S,Liu Y,et al.Outcomes of hybrid procedure for type B aortic dissection with an aberrant right subclavian artery[J].J Vasc Surg,2018,67(3):704-711.doi:10.1016/j.jvs.2017.07.124.
[19]Geisbüsch P,Kotelis D,Müller-Eschner M,et al.Complications after aortic arch hybrid repair[J].J Vasc Surg,2011,53(4):935-941.doi:10.1016/j.jvs.2010.10.053.
[20]Bünger CM,Kische S,Liebold A,et al.Hybrid aortic arch repair for complicated type B aortic dissection[J].J Vasc Surg,2013,58(6):1490-1496.doi:10.1016/j.jvs.2013.05.091.
[21]Xiang Y,Huang B,Zhao J,et al.The strategies and outcomes of left subclavian artery revascularization during thoracic endovascular repair for type B aortic dissection[J].Sci Rep,2018,8(1):9289.doi:10.1038/s41598-018-27588-7.
[22]Saouti N,Hindori V,Morshuis WJ,et al.Left subclavian artery revascularization as part of thoracic stent grafting[J].Eur J Cardiothorac Surg,2015,47(1):120-125.doi:10.1093/ejcts/ezu130.
[23]Piffaretti G,Pratesi G,Gelpi G,et al.Comparison of two different techniques for isolated left subclavian artery revascularization during thoracic endovascular aortic repair in zone 2[J].J Endovasc Ther,2018,25(6):740-749.doi:10.1177/1526602818802581.
[24]舒畅,罗明尧,李全明,等."烟囱"技术在累及主动脉弓部血管的动脉夹层腔内修复术中的应用[J].中国普通外科杂志,2010,19(12):1266-1270.
Shu C,Luo MY,Li QM,et al.Chimney grafts for endovascular repair of aortic dissection involving the aortic arch[J].Chinese Journal of General Surgery,2010,19(12):1266-1270.
[25]周静文,陈德基,林少芒,等.左锁骨下动脉“烟囱”技术在胸主动脉夹层腔内修复术中的应用[J].介入放射学杂志,2015,24(8):668-671.doi:10.3969/j.issn.1008-794X.2015.08.004.
Zhou JW,Chen DJ,Lin SM,et al.The application of "chimney" technique of left subclavian artery in performing endovascular repair procedure for Stanford type B aortic dissection[J].Journal of Interventional Radiology,2015,24(8):668-671.doi:10.3969/j.issn.1008-794X.2015.08.004.
[26]Rancic Z,Pfammatter T,Lachat M,et al.Periscope graft to extend distal landing zone in ruptured thoracoabdominal aneurysms with short distal necks[J].J Vasc Surg,2010,51(5):1293-1296.doi:10.1016/j.jvs.2009.11.076.
[27]罗明尧,舒畅,方坤,等.“HENDO“技术体系治疗主动脉弓部疾病[J].中国胸心血管外科临床杂志,2020,27(9):987-991.doi:10.7507/1007-4848.202002088.
Luo MY,Shu C,Fang K,et al.Aortic arch repair by “HENDO” technology clusters[J].Chinese Journal of Clinical Thoracic and Cardiovascular Surgery,2020,27(9):987-991.doi:10.7507/1007-4848.202002088.
[28]Zhu J,Dai X,Noiniyom P,et al.Fenestrated thoracic endovascular aortic repair using physician-modified stent grafts (PMSGs) in zone 0 and zone 1 for aortic arch diseases[J].Cardiovasc Intervent Radiol,2019,42(1):19-27.doi:10.1007/s00270-018-2079-9.
[29]Li X,Li Q,Zhang W,et al.Early experience and technical aspects of physician-modified fenestration in thoracic endovascular aortic repair for aortic arch pathologies[J].J Inter Med Res,2019.doi:10.1177/0300060519870903.[Online ahead of print]
[30]Hiramoto JS.Commentary:multiple chimney grafts for total endovascular revascularization of the visceral arteries in the setting of ruptured TAAA:inventive but let's wait for the smoke to clear on this one[J].J Endovas Ther,2010,17(2):222-223.doi:10.1583/09-2925C1.1.
[31]Donas KP,Torsello G,Austermann M,et al.Use of abdominal chimney grafts is feasible and safe:short-term results[J].J Endovasc Ther,2010,17(5):589-593.doi:10.1583/10-3083.1.
[32]Criado FJ.A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR):retrograde catheterization and stenting[J].J Endovasc Ther,2007,14(1):54-58.doi:10.1583/06-2010.1.
