随着外科手术技术的进步[1],肝脏手术的安全性已经得到了极大的提升,围术期病死率已经降至5%以下[2-3],但是术后并发症的发生情况仍不容乐观[4]。而严重的肝纤维化则会在很大程度上增加发生术后并发症的风险[5],如肝功能衰竭[6-7],腹腔积液[8]等。肝脏手术术前对于肝纤维化诊断的金标准仍为肝穿刺活检这一有创检查[9],而穿刺活检本身存在一定的风险[10]且可能存在样本误差[11-12]。目前用于患者的肝脏情况术前评估的其他途径通常包括:生化指标(如血常规、凝血功能及肝肾功能等)、Child-Pugh分级、吲哚氰绿清除实验[13]、门静脉超声检查[14]等这些途径的优点在于风险低且创伤小或者没有创伤,而缺点则是单一的检查仅仅只能从某一个角度间接地了解患者的肝脏情况,相对来说较为片面。而本研究的目的则是找出哪些检查对于肝纤维化的术前预测更有意义,以及能否联合其中某些检查建立一个肝纤维化的术前预测模型,以此来更加全面且准确地预测患者肝纤维化的严重程度,进一步降低肝切除术后发生并发症的风险。
1 资料与方法
1.1 一般资料
收集2018年9月—2019年12月期间共计106例患者的临床数据进行回顾性分析。纳入标准:⑴年龄18~75岁;⑵ 首次行肝切除术。排除标准:⑴ 术前肝功能Child-Pugh分级为C级;⑵ 胆道梗阻;⑶ 肾功能不全;⑷ 术前严重心、脑、肺等其他重要脏器疾病。本研究已经被湘雅医院伦理委员会批准(IRB[S]NO:202005056),所有患者均对本研究知情,且签署了知情同意书。
1.2 病理分级
取所有患者手术标本中的健康肝组织进行研究,且每个样本都由2名经验丰富的病理科医生依据Laennec病理分级标准[15]进行分级。将Laennec分级为0、1、2和3级的患者归为无或低级别肝纤维化组;Laennec分级为4级的患者则被归为高级别肝纤维化组。所有病理切片都经过HE染色并由Olympus BX53显微镜进行检查。
1.3 研究设计
将患者依据肝纤维化程度分为无或低级别肝纤维化组和高级别肝纤维化组。第一步,先将两组患者的所有检查指标进行单因素分析,以筛选出其中有统计学差异的指标;第二步,再将以上指标纳入多因素Logistic回归分析,筛选出独立预测指标并建立预测模型;第三步,建立受试者工作特征(ROC)曲线,评价独立预测指标及综合预测模型的预测效果。
1.4 检查指标
所有患者均在入院后第2 天清晨空腹采外周静脉血送检,生化指标包括白细胞(WBC)、血小板(PLT)、白蛋白(ALB)、血清总胆红素(TBIL)、凝血酶原时间(PT)、血肌酐(Cr)和乙型肝炎表面抗原(HBsAg)。结合生化指标及临床表现得到Child-Pugh分级。吲哚氰绿清除试验记录患者吲哚氰绿15分钟滞留率(ICG15)。门静脉超声检查记录患者的门静脉宽度及流速。
1.5 统计学处理
计量资料采用均数±标准差( ±s)表示,组间比较采用t 检验;计数资料采用例数百分率[n(%)]表示,组间比较采用χ2验。将差异有统计学意义的指标纳入二分类Logistic回归分析,筛选出独立预测指标并利用其建立回归模型,采用ROC曲线评价预测模型诊断效率。使用SPSS 26分析全部数据,以P<0.05为差异有统计学意义。
±s)表示,组间比较采用t 检验;计数资料采用例数百分率[n(%)]表示,组间比较采用χ2验。将差异有统计学意义的指标纳入二分类Logistic回归分析,筛选出独立预测指标并利用其建立回归模型,采用ROC曲线评价预测模型诊断效率。使用SPSS 26分析全部数据,以P<0.05为差异有统计学意义。
2 结 果
2.1 单因素分析结果
本研究共纳入106例患者,其中男97例;女9例。所有患者均因肝脏恶性肿瘤行肝脏切除术,其中肝细胞癌(HCC)患者97例;胆管细胞癌(ICC)患者9例。依据肝纤维化严重程度将其中50例归入无或低级别肝纤维化组;56例归入高级别肝纤维化组。单因素组间比较结果显示,两组之间WBC、PLT、PT、Cr、ICG15、门静脉宽度及门静脉流速的差异具有统计学意义(均P<0.05)(表1)。
表1 两组患者相关指标的单因素分析
Table1 Univariate analysis of related indicators of the two groups of patients
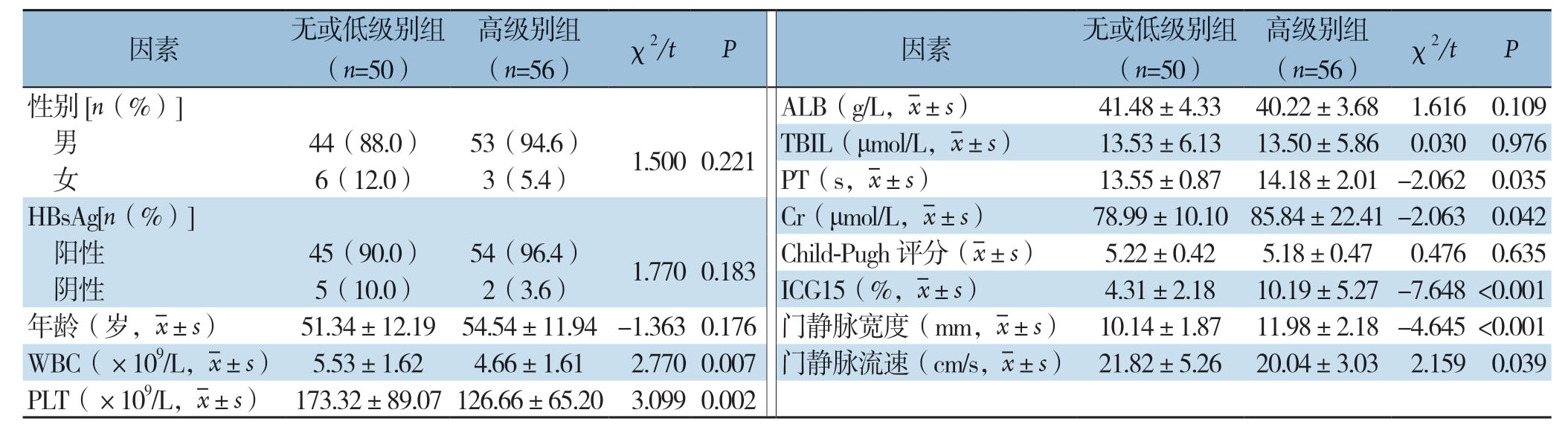
因素 无或低级别组(n=50)高级别组(n=56) χ2/t P 因素 无或低级别组(n=50)高级别组(n=56) χ2/t P性别[n(%)]ALB(g/L,images/BZ_82_483_743_511_786.png±s) 41.48±4.33 40.22±3.68 1.616 0.109男44(88.0) 53(94.6) 1.500 0.221 TBIL(μmol/L,images/BZ_82_483_743_511_786.png±s) 13.53±6.13 13.50±5.86 0.030 0.976女6(12.0) 3(5.4)PT(s,images/BZ_82_483_743_511_786.png±s) 13.55±0.87 14.18±2.01 -2.062 0.035 HBsAg[n(%)]Cr(μmol/L,images/BZ_82_483_743_511_786.png±s) 78.99±10.10 85.84±22.41 -2.063 0.042阳性 45(90.0) 54(96.4) 1.770 0.183 Child-Pugh 评分(images/BZ_82_483_743_511_786.png±s) 5.22±0.42 5.18±0.47 0.476 0.635阴性 5(10.0) 2(3.6)ICG15(%,images/BZ_82_483_743_511_786.png±s) 4.31±2.18 10.19±5.27 -7.648 <0.001年龄(岁,images/BZ_82_483_743_511_786.png±s) 51.34±12.19 54.54±11.94 -1.363 0.176门静脉宽度(mm,images/BZ_82_483_743_511_786.png±s) 10.14±1.87 11.98±2.18 -4.645 <0.001 WBC(×109/L,images/BZ_82_483_743_511_786.png±s) 5.53±1.62 4.66±1.61 2.770 0.007门静脉流速(cm/s,images/BZ_82_483_743_511_786.png±s) 21.82±5.26 20.04±3.03 2.159 0.039 PLT(×109/L,images/BZ_82_483_743_511_786.png±s) 173.32±89.07 126.66±65.20 3.099 0.002
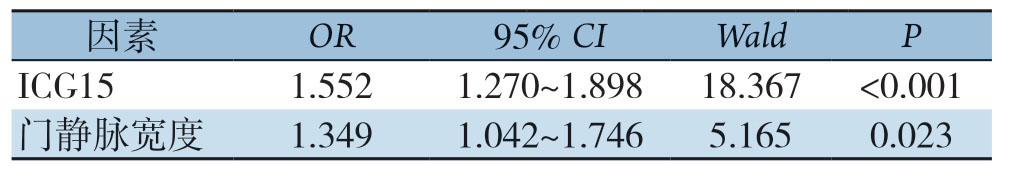
2.2 多因素Logistic回归分析结果
将上述在单因素分析中筛选出来具有统计学差异的指标纳入多因素二分类Logistic回归分析中,并通过逐步向前法最终得到独立预测指标为ICG15与门静脉宽度(均P<0.05)(表2),同时可建立基于以上两项检查指标的回归模型:Logit(P)=-6.026+0.44×ICG15+0.299×门静脉宽度。
2.3 预测价值分析
分别对ICG15、门静脉宽度以及联合以上两项指标的回归模型建立预测高级别肝纤维化的ROC曲线。结果显示:ICG15的曲线下面积(AUC)为0.86,截断值为5.05时,敏感度为85.7%,特异度为72%。门静脉宽度的AUC为0.73,截断值为10.75时,敏感度为71.4%,特异度为66%。综合预测模型的AUC为0.88,截断值为0.36时,敏感度为89.3%,特异度为74%。由此可见,ICG15与门静脉宽度这两项检查指标均对高级别肝纤维化有良好的预测效果,而综合预测模型相比单项检查则能更好地反映患者肝纤维化的严重程度(图1)。
表2 多因素Logistic回归分析
Table2 Multivariate Logistic regression analysis
因素 OR 95% CI Wald P ICG15 1.552 1.270~1.898 18.367 <0.001门静脉宽度 1.349 1.042~1.746 5.165 0.023
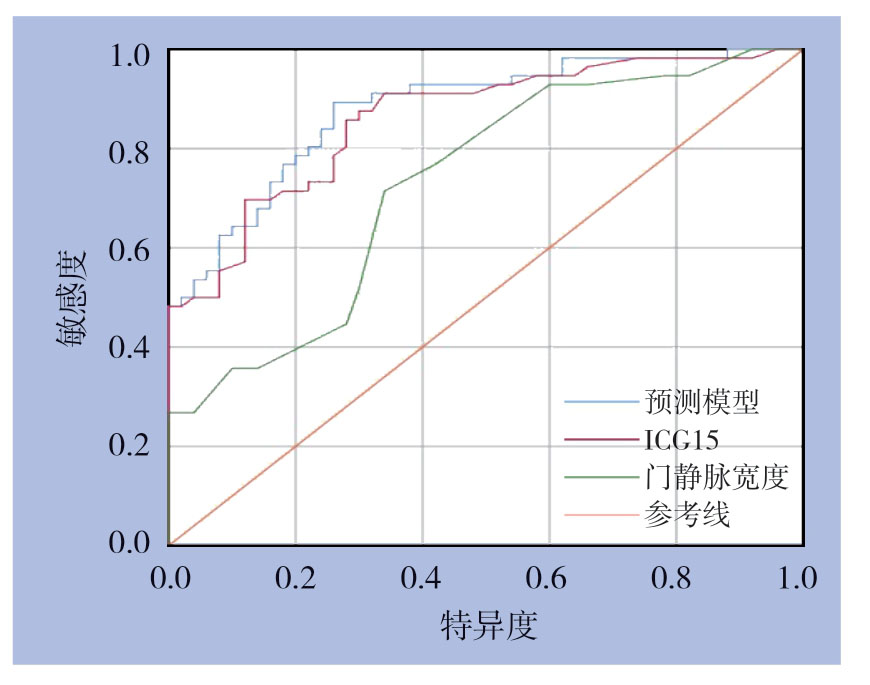
图1 综合预测模型、ICG15 及门静脉宽度预测高级别肝纤维化的ROC曲线
Figure1 ROC curves of the integrated prediction model,ICG15 and portal vein width to predict high-stage fibrosis
3 讨 论
对于肝脏恶性肿瘤和部分良性肿瘤而言,外科手术仍是一线治疗方案[16-17],而术后并发症的发生则极大地影响了患者的恢复情况和生活质量,同时浪费了宝贵的医疗资源[18-19]。如何降低肝切除术后并发症的发生概率便成为了近年来重点关注的议题[20-21],肝纤维化的严重程度则是其最为关键的影响因素之一[22]。肝纤维化越严重意味着肝切术后残余肝的功能越差,肝功能恢复所需要的时间也越长,发生术后肝功能衰竭等严重并发症的可能性也就越大[23-24]。因此,只有通过准确的术前预测,才可以依据不同患者肝纤维化的严重程度,制定出对患者最有利的个性化治疗方案,包括如手术切除范围、手术方式,甚至是整体治疗方案上决策[25]。力争在保证治疗效果的同时,尽可能地降低肝切除术后并发症的发生率。
本研究依据患者的手术病理切片资料,以Laennec病理分级为标准,将所有患者分为无或低级别肝纤维化和高级别肝纤维化两组。在第一步中将目前最为常用的检查指标纳入组间对比。结果显示两组间WBC、PLT、PT、Cr、ICG15、门静脉宽度及门静脉流速具有统计学差异。随后在第二步中将以上差异指标纳入多因素回归分析,得到独立预测指标为ICG15与门静脉宽度,并以此为基础建立综合两项指标的预测模型。最后通过建立预测高级别肝纤维化的ROC曲线,验证了ICG15与门静脉宽度这两个独立指标对高级别肝纤维化具有良好的预测效果,而联合两项指标的综合预测模型的预测效果更是优于独立指标。
常规的生化检查及以其为基础的Child-Pugh分级虽然简单易行且运用广泛,但是也存在明显的局限性。第一,Child-Pugh分级标准中关于临床症状(如腹水及肝性脑病)的评判,容易受到主观因素的影响;其次,Child-Pugh分级对于轻度肝纤维患者的评估意义有限,而对于肝硬化失代偿期患者的评估效果更好;另外,由于我们也很难通过常规的生化检查结果和Child-Pugh分级来了解患者肝脏的储备功能,所以其对于肝切除术前的风险评估价值也相对有限[23]。吲哚氰绿清除实验则是利用了肝细胞对吲哚氰绿选择性摄取且不参与肝肠循环的特性,而由此所得到的指标ICG15则可以用于反映肝脏的储备功能、评估手术风险[26-27]。本研究得到ICG15为高级别肝纤维化的独立预测指标,与Moller等[28]的研究结果相符。肝脏纤维化程度越严重,门静脉血流受阻也就越明显、门静脉压力也就越高[29],而无创超声检查则可以很好地反映门静脉的宽度及血流速度等情况。如前文所述,门静脉宽度是另一项高级别肝纤维化的独立预测指标,与以往的研究结果一致[30]。
综上所述,ICG15与门静脉宽度均可有效地预测高级别肝纤维化。在此基础之上,综合预测模型在ROC曲线分析中的AUC、敏感度和特异度均高于独立指标,说明联合ICG15与门静脉宽度的综合预测模型能对患者肝纤维化严重程度进行更为准确的预测。更可以此为基础,获得更加真实、有效且全面的术前评估,尽可能地将患者肝切除术后并发症的发生概率降至最低。
[1]张伟,陈孝平.肝脏外科的发展现状及展望[J].中华外科杂志,2019,57(7):488–493.doi:10.3760/cma.j.issn.0529–5815.2019.07.002.
Zhang W,Chen XP.Current status and prospect of liver surgery[J].Chinese Journal of Surgery,2019,57(7):488–493.doi:10.3760/cma.j.issn.0529–5815.2019.07.002.
[2]Mullen JT,Ribero D,Reddy SK,et al.Hepatic insufficiency and mortality in 1059 noncirrhotic patients undergoing major hepatectomy[J].J Am Coll Surg,2007,204(5):854–864.doi:10.1016/j.jamcollsurg.2006.12.032.
[3]Poon RT,Fan ST,Lo CM,et al.Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases:analysis of 1222 consecutive patients from a prospective database[J].Ann Surg,2004,240(4):698–710.doi:10.1097/01.sla.0000141195.66155.0c.
[4]Ishizawa T,Hasegawa K,Kokudo N,et al.Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma[J].Arch Surg,2009,144(1):46–51.doi:10.1001/archsurg.2008.511.
[5]Hackl C,Schlitt H J,Renner P,et al.Liver surgery in cirrhosis and portal hypertension[J].World J Gastroenterol,2016,22(9):2725–2735.doi:10.3748/wjg.v22.i9.2725.
[6]计勇,甄作均,苏树英,等.肝移植治疗肝硬化肝癌切除后肝功能衰竭[J].中国普通外科杂志,2002,11(9):564–565.doi:10.3969/j.issn.1005–6947.2002.09.018.
Ji Y,Zhen ZJ,Su SY,et al.Liver transplantation for hepatocarcinoma patients with cirrhosis and hepatic failure after partial hepatectomy[J].Chinese Journal of General Surgery,2002,11(9):564–565.doi:10.3969/j.issn.1005–6947.2002.09.018.
[7]van Mierlo KM,Schaap FG,Dejong CH,et al.Liver resection for cancer:New developments in prediction,prevention and management of postresectional liver failure[J].J Hepatol,2016,65(6):1217–1231.doi:10.1016/j.jhep.2016.06.006.
[8]Piano S,Tonon M,Angeli P.Management of ascites and hepatorenal syndrome[J].Hepatol Int,2018,12(Suppl 1):122–134.doi:10.1007/s12072–017–9815–0.
[9]中华医学会肝病学分会,中华医学会消化病学分会,中华医学会感染病学分会.肝纤维化诊断及治疗共识(2019年)[J].临床肝胆病杂志,2019,35(10):2163–2172.doi:10.3969/j.issn.1001–5256.2019.10.007.
Society of Hepatology of Chinese Medical Association,Society of Gastroenterology of Chinese Medical Association,Society of Infectious Diseases of Chinese Medical Association.Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J].Journal of Clinical Hepatology,2019,35(10):2163–2172.doi:10.3969/j.issn.1001–5256.2019.10.007.
[10]范平,江军,冯晓峰,等.500例经皮肝穿刺活检并发症分析[J].中华消化杂志,2004,24(7):426.doi:10.3760/j.issn:0254–1432.2004.07.012.
Fan P,Jiang J,Feng XF,et al.Analysis of complications of percutaneous liver biopsy in 500 cases[J].Chinese Journal of Digestion,2004,24(7):426.doi:10.3760/j.issn:0254–1432.2004.07.012.
[11]Rousselet MC,Michalak S,Dupré F,et al.Sources of variability in histological scoring of chronic viral hepatitis[J].Hepatology.2005,41(2):257–264.doi:10.1002/hep.20535.
[12]胡锡琪.慢性肝炎肝纤维化病理分期和评估[J].中华肝脏病杂志,2008,16(3):169–170.doi:10.3321/j.issn:1007–3418.2008.03.004.
Hu XQ.Histopathological staging and assessment of liver fibrosis in chronic hepatitis patients[J].Chinese Journal of Hepatology,2008,16(3):169–170.doi:10.3321/j.issn:1007–3418.2008.03.004.
[13]秦华,万红,吴晓庆,等.吲哚菁绿清除试验对肝硬化及肝衰竭患者肝脏储备功能的评估及预后的判断[J].中华肝脏病杂志,2015,23(7):540–542.doi:10.3760/cma.j.issn.1007–3418.2015.07.015.
Qin H,Wan H,Wu XQ,et al.Indocyanine green clearance test assessment of liver reserve function and estimation of prognosis in patients with cirrhosis and liver failure[J].Chinese Journal of Hepatology,2015,23(7):540–542.doi:10.3760/cma.j.issn.1007–3418.2015.07.015.
[14]郑荣琴,吕明德,周元平.门静脉血流动力学改变与肝纤维化程度的相关性[J].中山医科大学学报,1999,20(2):141.doi:10.3321/j.issn:1672–3554.1999.02.017.
Zheng RQ,Lu MD,Zhou YP.Correlation between changes in hemodynamics of the portal vein and severity of liver fibrosis[J].Academic Journal of Sun Yat-Sen University of Medical Sciences,1999,20(2):141.doi:10.3321/j.issn:1672–3554.1999.02.017.
[15]Wang W,Li J,Pan R,et al.Association of the Laennec staging system with degree of cirrhosis,clinical stage and liver function[J].Hepatol Int,2015,9(4):621–626.doi:10.1007/s12072–015–9648–7.
[16]中华外科学会肝脏外科研究组.原发性肝癌外科治疗方法的选择[J].中国普通外科杂志,2005,14(2):81–83.doi:10.3969/j.issn.1005–6947.2005.02.001.
The Hepatiic Surgery Research Group of Chinese Surgery.Selection of surgery therapeutic modalities for primary hepatocellular carcinoma[J].Chinese Journal of General Surgery,2005,14(2):81–83.doi:10.3969/j.issn.1005–6947.2005.02.001.
[17]Hammond JS,Guha IN,Beckingham IJ,et al.Prediction,prevention and management of postresection liver failure[J].Br J Surg,2011,98(9):1188–1200.doi:10.1002/bjs.7630.
[18]Torzilli G,Makuuchi M,Inoue K,et al.No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients:is there a way? A prospective analysis of our approach[J].Arch Surg,1999,134(9):984–992.doi:10.1001/archsurg.134.9.984.
[19]Chen MS,Li JQ,Zheng Y,et al.A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma[J].Ann Surg,2006,243(3):321–328.doi:10.1097/01.sla.0000201480.65519.b8.
[20]黄莚庭.努力降低肝胆外科手术并发症[J].肝胆外科杂志,2002,10(6):401–402.doi:10.3969/j.issn.1006–4761.2002.06.001.
Huang YT.Efforts to reduce the complications in hepatobiliary surgery[J].Journal of Hepatobiliary Surgery,2002,10(6):401–402.doi:10.3969/j.issn.1006–4761.2002.06.001.
[21]Grazi GL,Ercolani G,Pierangeli F,et al.Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value[J].Ann Surg,2001,234(1):71–78.doi:10.1097/00000658–200107000–00011.
[22]Ercolani G,Grazi GL,Ravaioli M,et al.Liver resection for hepatocellular carcinoma on cirrhosis:univariate and multivariate analysis of risk factors for intrahepatic recurrence[J].Ann Surg,2003,237(4):536–543.doi:10.1097/01.SLA.0000059988.22416.F2.
[23]Schneider PD.Preoperative assessment of liver function[J].Surg Clin North Am,2004,84(2):355–373.doi:10.1016/S0039–6109(03)00224-X.
[24]Huang ZY,Liang BY,Xiong M,et al.Severity of cirrhosis should determine the operative modality for patients with early hepatocellular carcinoma and compensated liver function[J].Surgery,2016,159(2):621–631.doi:10.1016/j.surg.2015.09.002.
[25]Kokudo T,Hasegawa K,Shirata C,et al.Assessment of Preoperative Liver Function for Surgical Decision Making in Patients with Hepatocellular Carcinoma[J].Liver Cancer,2019,8(6):447–456.doi:10.1159/000501368.
[26]Mullin EJ,Metcalfe MS,Maddern GJ.How much liver resection is too much?[J].Am J Surg,2005,190(1):87–97.doi:10.1016/j.amjsurg.2005.01.043.
[27]邱越,熊杰,彭英,等.吲哚氰绿清除试验对丙肝肝硬化脾切除术后门静脉血栓形成风险的预测价值[J].中国普通外科杂志,2014,23(1):87–90.doi:10.7659/j.issn.1005–6947.2014.01.017.
Qiu Y,Xiong J,Peng Y,et al.Value of indocyanine green clearance test for predicting the risk of portal vein thrombosis after splenectomy in patients with hepatitis C cirrhosis[J].Chinese Journal of General Surgery,2014,23(1):87–90.doi:10.7659/j.issn.1005–6947.2014.01.017.
[28]Møller S,la Cour Sibbesen E,Madsen JL,et al.Indocyanine green retention test in cirrhosis and portal hypertension:Accuracy and relation to severity of disease[J].J Gastroenterol Hepatol,2019,34(6):1093–1099.doi:10.1111/jgh.14470.
[29]Simonetto DA,Liu M,Kamath PS.Portal Hypertension and Related Complications:Diagnosis and Management[J].Mayo Clin Proc,2019,94(4):714–726.doi:10.1016/j.mayocp.2018.12.020.
[30]耿繁淳,王艳玲,李清州.肝硬化病人门脉宽度的临床意义[J].中国超声医学杂志,1992,(S1):25–26.
Geng FC,Wang YL,Li QZ.Clinical significance of the width of portal vein in patients with liver cirrhosis[J].Chinese Journal of Ultrasound in Medicine,1992(S1):25–26.
