尽管针对胰腺癌的诊疗技术不断提高,但胰腺癌的预后仍然很差,5年生存率低于5%[1-2]。由于胰体尾癌患者早期缺乏特异性症状及体征,在诊断时仅15%~20%被定义为可切除性肿瘤,而大多数因肿瘤发生远处转移或侵犯腹腔干(CA)、肝总动脉(CHA)、肠系膜上动脉(SMA)等周围血管被定义为不切除性肿瘤[3-8]。尽管近年来化疗及放疗等治疗为局部进展期胰体尾癌患者带来较好的疗效,但R 0切除性手术仍然是唯一可能使患者治愈或带来较长生存期的治疗方法[9-12]。
联合脾脏的胰腺远端切除术(distal pancreatectomy,DP)是治疗胰腺体部和尾部肿瘤的标准手术方案[8]。Lyon Appleby于1953年首次报道了远端胰腺切除联合腹腔干切除术及全胃切除治疗局部进展期胃癌患者[13]。而Nimura等[14]1976年首次应用改良Appleby术即保留胃的联合腹腔干切除胰体尾癌根治术(distal pancreatectomy with en bloc celiac axis resection,DP-CAR)用于治疗CA及其分支受累的局部进展期胰体尾癌患者,此后,DP-CAR被越来越多的外科医生应用于局部进展期胰体尾癌患者的治疗。DP-CAR为胰体尾癌侵犯CA及CHA的患者提供了手术机会,显著提高了R 0的切除率,延长了生存期,并缓解了疼痛,提高了患者的生活质量[15-28],但因其DP-CAR术式的复杂性和较高的术后病死率及并发症发生率,其临床疗效及安全性仍存在诸多争议。
高质量的Meta被认为是获得证据的关键工具之一,可为临床医师的诊疗提供决策依据[29-31]。虽然有1篇关于对比DP-CAR与DP疗效的Meta分析于2016年发表[32],但其纳入的18篇文献,均发表于2014年前,且每个研究样本量均较小,仅5篇为DP-CAR与DP的对比性研究,所得结果局限性大,可靠性差。为了获得更加可靠的临床决策依据,本研究进行了更为全面的文献检索及筛选,对DP-CAR在局部进展期胰体尾癌的疗效和安全性进行全面详细的Meta分析,为临床工作提供决策依据参考。
1 资料与方法
1.1 检索策略
计算机检索PubMed、Cochran Library、EMBASE、Web of Science、CNKI、万方等数据库。英文检索词包括:Celiac Axis、Appleby、Pancreatic Cancer、Distal Pancreatectomy;中文检索词包括:改良Appleby、腹腔干切除、胰体尾癌根治术等。采取主题词与自由词相结合方式进行文献检索,由2名研究员独立进行,检索年限为各数据库建库至2019年6月。
1.2 纳入与排除标准
纳入标准:⑴ 原发癌为胰体尾癌;⑵ D PCAR和DP的比较性研究,其患者的年龄、性别、种族、国籍等不受限制;⑶ 至少包含一个与研究相关的结局指标;⑷ 对同一研究者或单位重复发表的研究,仅纳入质量较高的或近期发表的研究。排除标准:⑴ 伴发其他类型的恶性肿瘤;⑵ 病例数<5例;⑶ 非原始数据;⑷ 个案报道、审查、信、会议等研究。
1.3 资料的提取及质量评价
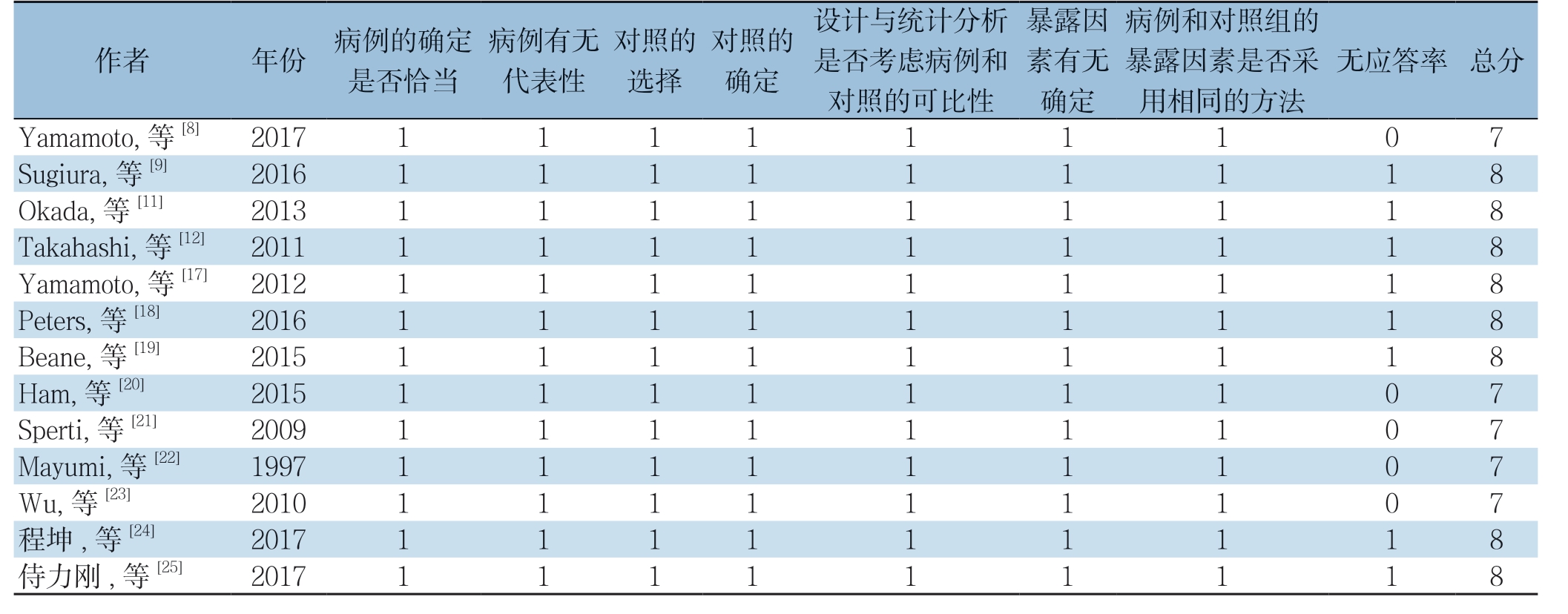
由2名研究员通过阅读题目、摘要及全文等独立进行文献筛选、提取数据并交叉核对,如遇到分歧,则由第3方或讨论解决。纳入研究资料提取的内容:⑴ 一般资料:作者、发表年份、国家、研究时间、研究类型、样本量、患者的年龄、性别;⑵ 结局指标:术后生存期(1、2、3年生存率)、R0切除率、近期病死率、术后并发症(包括胰瘘、胃排空延迟、再次手术率)、手术时间、术中失血量。采用NOS量表(Newcastle-Ottawa Scale)对纳入的非随机对照研究(NRCS)进行文献质量评价 [33]。
1.4 数据统计分析
采用RevMan 5.3软件对数据进行处理分析。计量资料及计数资料分别采用均数差(mean difference,MD)、相对危险度(risk ratio,RR)及95%的置信区间(confidence interval,CI)分析统计量,当P<0.05,认为两组间的差异具有统计学意义。通过χ2检验对纳入研究的各指标进行异质性检验,同时结合I 2及P值判断异质性的大小。若各研究结果间无明显异质性(P>0.1,I 2≤50%),则采用固定效应模型(fixed effects model)合并效应值;反之,若各研究结果间存在明显异质性(P≤0.1,I2>50%),则进行敏感性分析,分析异质性来源,并采用随机效应模型(random effects model)进行Meta分析[34-35]。当纳入文献的数量>10时,通过绘制漏斗图来评价发表偏倚。
2 结 果
2.1 文献筛选与纳入研究的特点
初步检索到1 064篇相关文献,通过查重、浏览题目摘要、阅读全文后筛选出符合纳入标准的13篇文献[8-9, 11-12, 17-25],其均为NRCS(1篇为前瞻性研究,12篇为回顾性研究;2篇为多中心研究,11篇为单中心研究),文献筛选流程见图1。共纳入1 219例患者,DP-CAR组237例,DP组982例,纳入文献的基本特征及质量评价见表1-2。
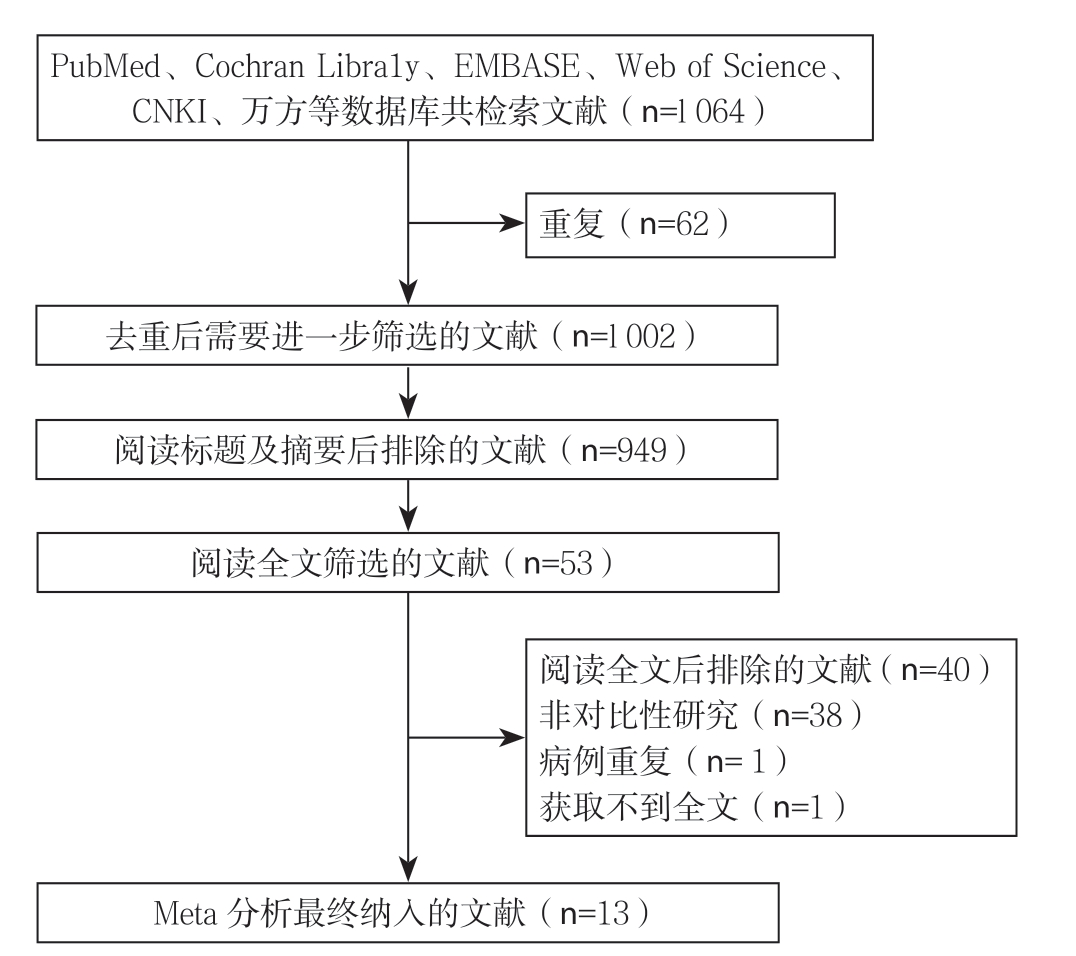
图1 文献筛选流程
Figure 1 Literature screening process
表1 纳入研究的基本特征
Table 1 The general characteristics of the included studies
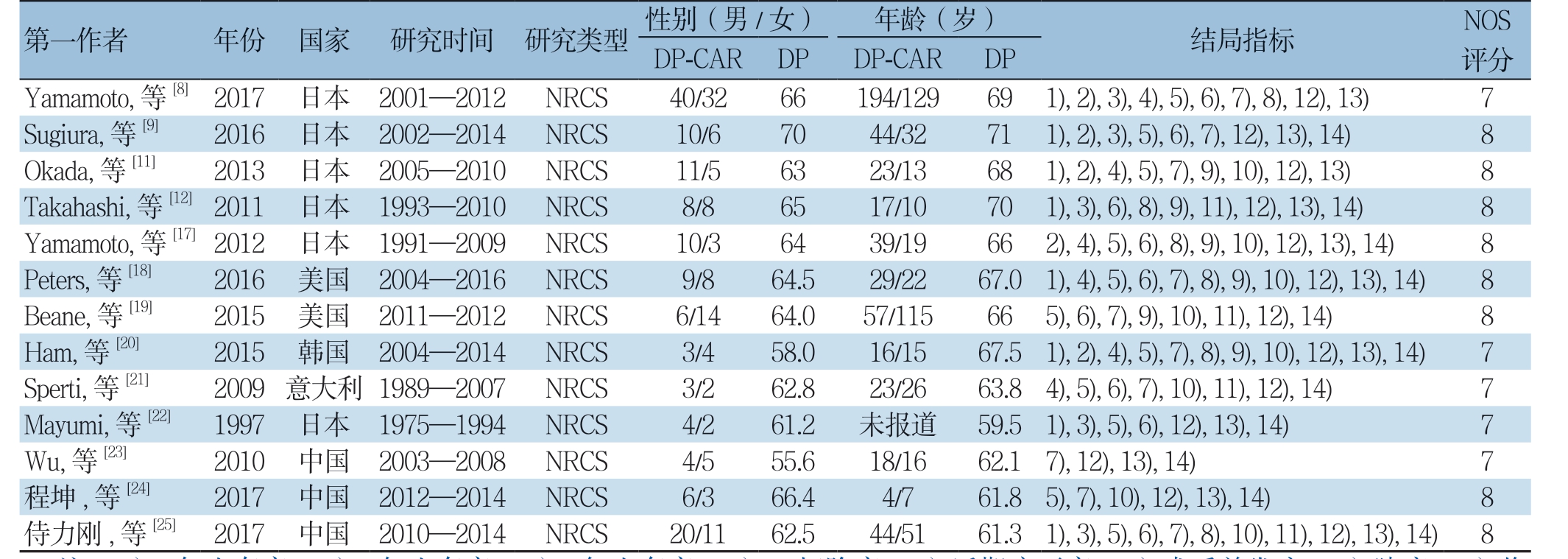
注:1)1 年生存率;2)2 年生存率;3)3 年生存率;4)R0 切除率;5)近期病死率;6)术后并发症;7)胰瘘;8)临床相关性胰瘘;9)胃排空延迟;10)腹腔出血;11)再次手术率;12)手术时间;13)失血量;14)住院时间
Note: 1) 1-year survival rate; 2) 2-year survival rate; 3) 3-year survival rate; 4) R0 resection rate; 5) Early mortality rate; 6) Postoperative complications; 7) Pancreatic fistula; 8) Clinically relevant pancreatic fistula; 9) Delayed gastric emptying; 10) Intra-abdominal hemorrhage;11) Reoparation rate; 12) Operative time; 13) Amount of blood loss; 14) Length of hospital stay
表2 纳入研究偏倚质量评价(分)
Table 2 Quality assessment of the included studies (score)
2.2 Meta 分析结果
2.2.1 生存率 有8 项研究[8-9, 11-12, 18, 20, 22, 25]报道了1 年生存率,5 项研究[8-9, 11, 17, 20]报道了2 年生存率,5 项研究[8-9, 12, 22, 25]报道了3 年生存率。DPCAR 组患者术后1、2、3 年生存率分别为55.8%(101/181)、30.6%(38/124)、19.6%(28/141),DP 组分别为77.1%(507/658)、48.3%(253/524)、26.5%(143/540)。Meta 分 析 结 果 显 示,DPCAR 组的1、2、3 年的生存率均低于DP,但差异无 统 计 学 意 义(RR=0.86,95% CI=0.63~1.18,P=0.36;RR=0.70,95% CI=0.45~1.10,P=0.12;RR=0.93,95% CI=0.51~1.70,P=0.82)(图2)。
2.2.2 R0 切除率 6 项研究[8, 11, 17-18, 20-21]报道了R0 切 除 率,DP-CAR 及DP 组R0 切 除 率 分 别 为62.7%(79/126)、79.2%(434/548)。结 果 显示R0 切除率在两组间的差异具有统计学意义,且DP-CAR 组的R0 切除率明显低于DP 组(RR=0.78,95% CI=0.68~0.90,P=0.000 6)(图3)。
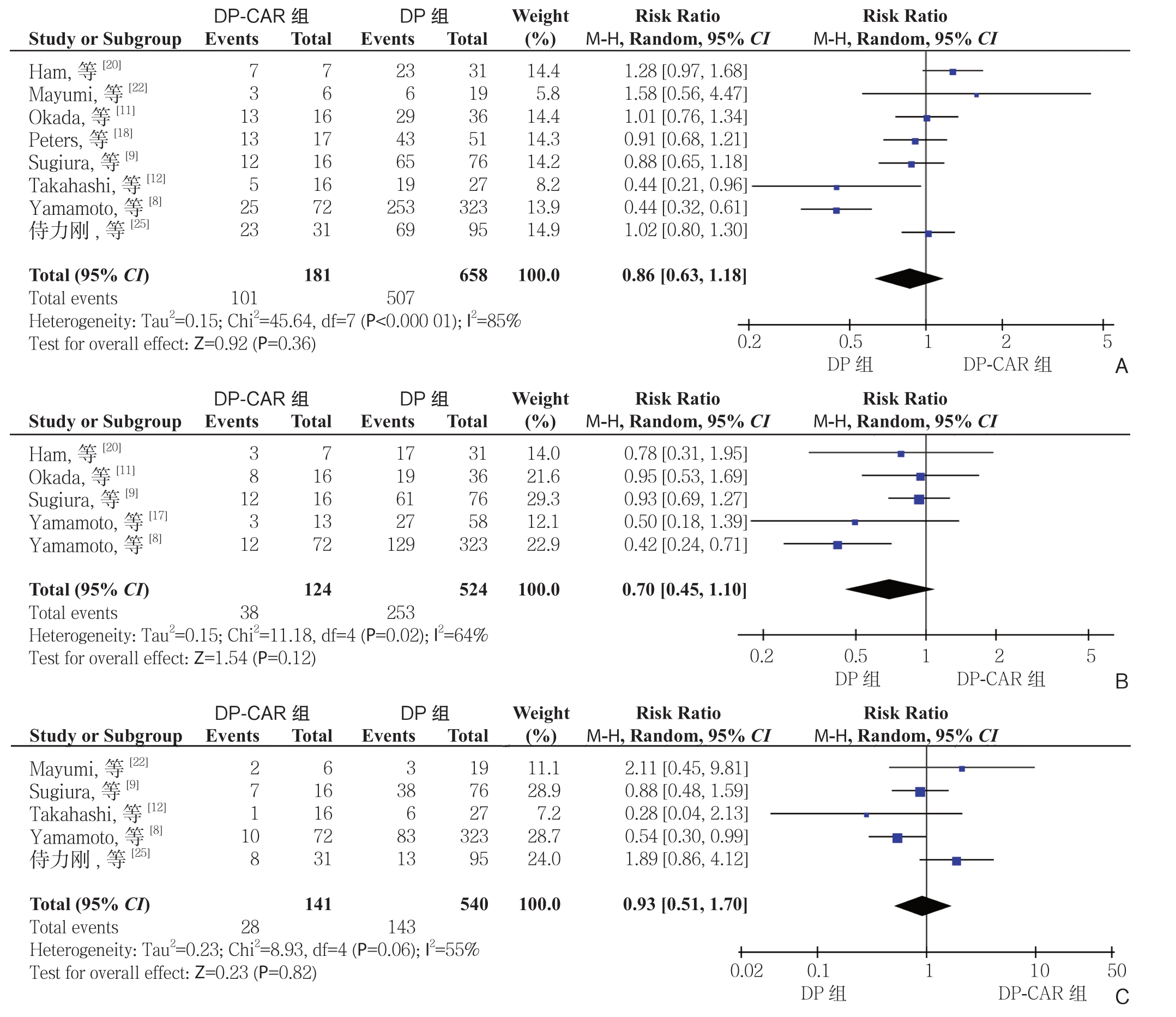
图2 DP-CAR 组与DP 组术后生存率比较 A:1 年生存率;B:2 年生存率;C:3 年生存率
Figure 2 Comparison of the survival rates between DP-CAR group and DP group A: 1-year survival rate; B: 2-year survival rate; C: 3-year survival rate
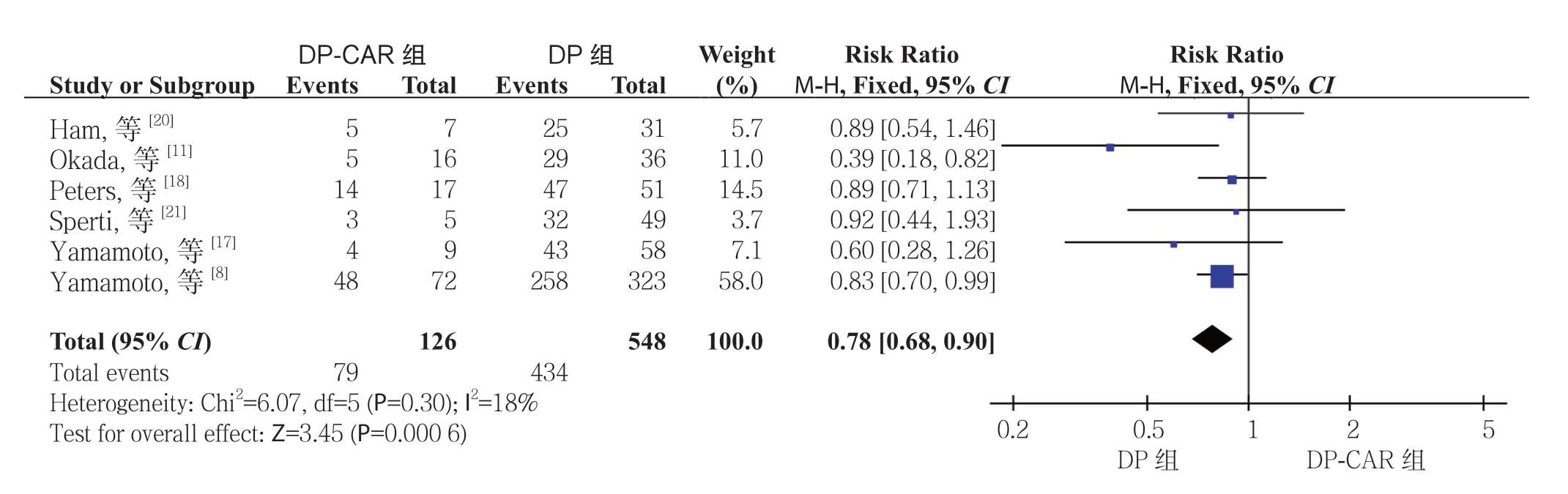
图3 DP-CAR 组与DP 组R0 切除率比较
Figure 3 Comparison of the R0 resection rates between DP-CAR group and DP group
2.2.3 近期病死率 11 项研究[8-9, 11, 17-22, 24-25]报道了近期病死率,有6 项研究[9, 11, 17-18, 22, 24]发生率为零。Meta 分析结果显示DP-CAR 组的术后近期病死率明显高于DP 组,差异具有统计学意义(RR=4.91,95% CI=1.90~12.67,P=0.001)(图4)。
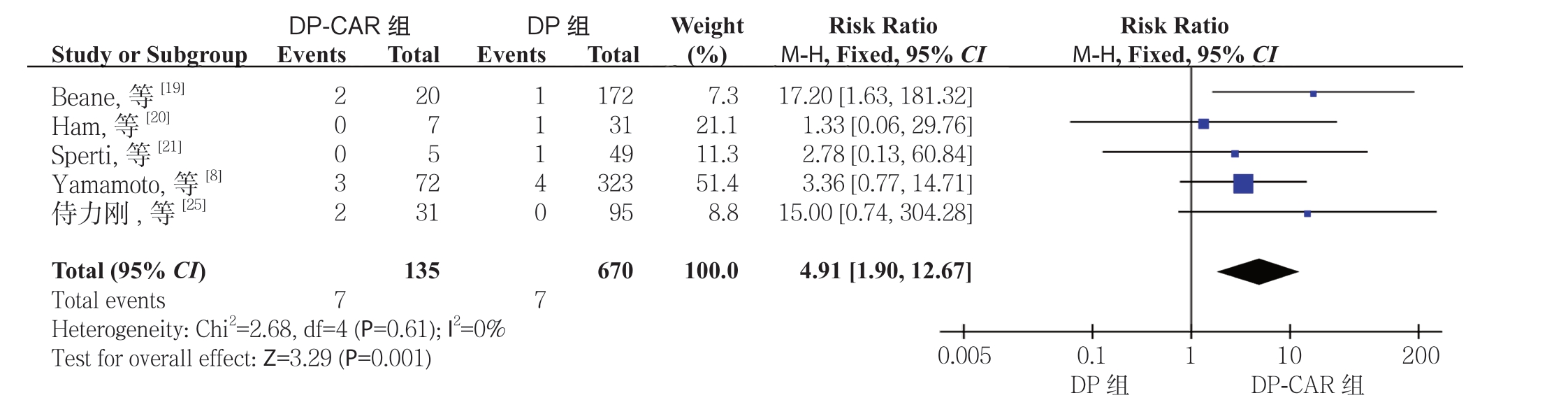
图4 DP-CAR 组与DP 组术后近期病死率比较
Figure 4 Comparison of the early postoperative mortality rates between DP-CAR group and DP group
2.2.4 术后并发症 胰瘘根据ISGPF 定义[36]。10 项研究[8-9, 11, 18-21, 23-25]报道了胰瘘,6 项研究[8, 12, 17-18,20, 25]报道了临床相关性胰瘘,其DP-CAR 组发生率分别为36.1%(73/202)、30.1%(47/156),而在DP 组分别30.8%(271/878)、20.5%(120/585)。Meta 分析结果显示,DP-CAR 组与DP 组的临床相关性胰瘘的发生率有统计学差异(RR=1.57,95% CI=1.19~2.08,P=0.002),而 胰 瘘 发 生 率无 统 计 学 差 异(RR=1.12,95% CI=0.92~1.36,P=0.27)(图5)。7 项 研 究[11-12 ,17-20, 24]报 道了胃排空延迟的发生率,DP-CAR 组为11.2%(11/98),而DP 组 为4.4%(17/386)。Meta分析结果显示DP-CAR 组的胃排空延迟的发生率明显高于DP 组,且具有统计学意义(RR=2.78,95% CI=1.33~5.81,P=0.007)(图6)。
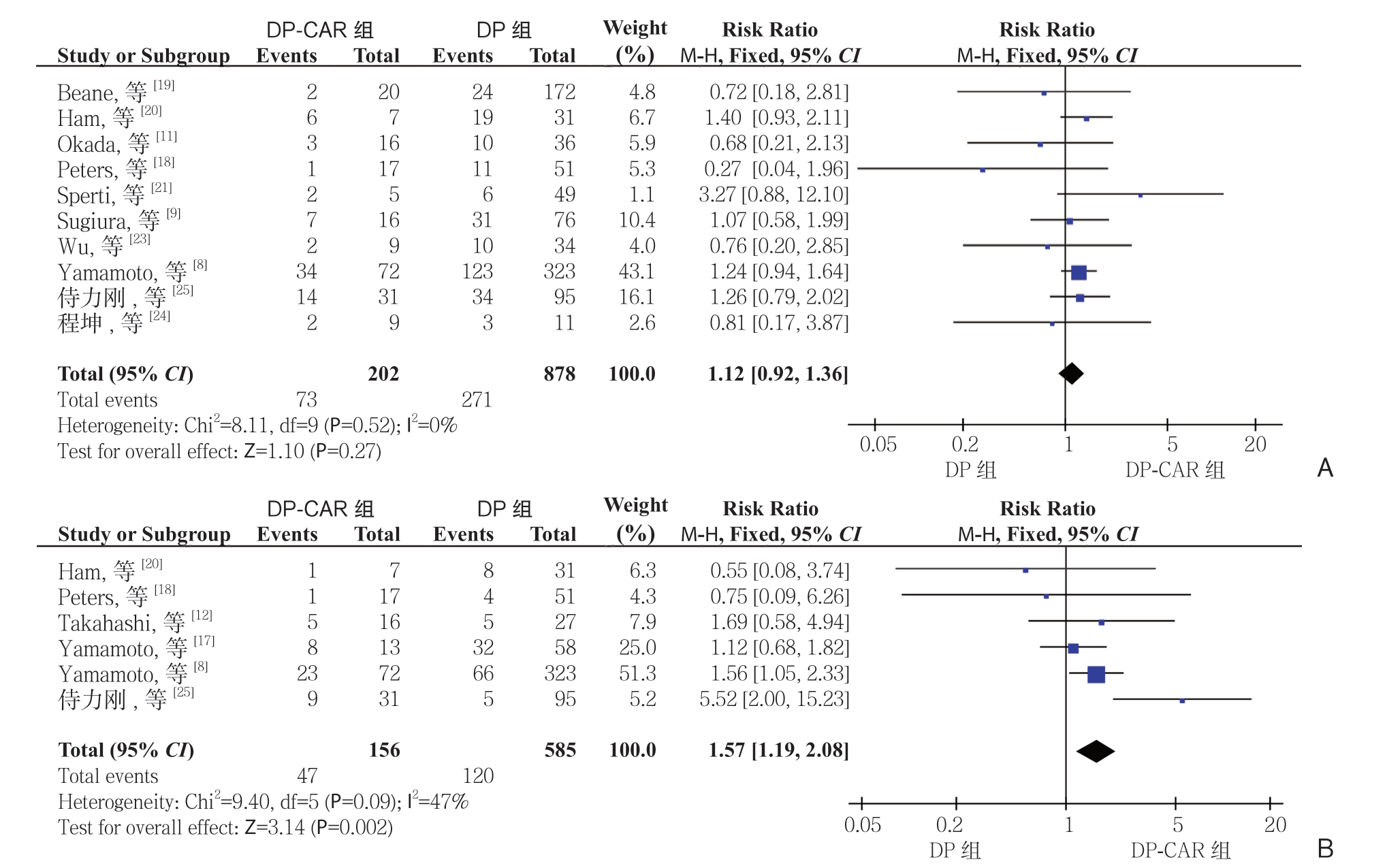
图5 DP-CAR 组与DP 组术后胰瘘发生率比较 A:胰瘘;B:临床相关性胰瘘
Figure 5 Comparison of the incidence rates of postoperative pancreatic fistula between DP-CAR group and DP group A: Pancreatic fistula; B: Clinically relevant pancreatic fistula
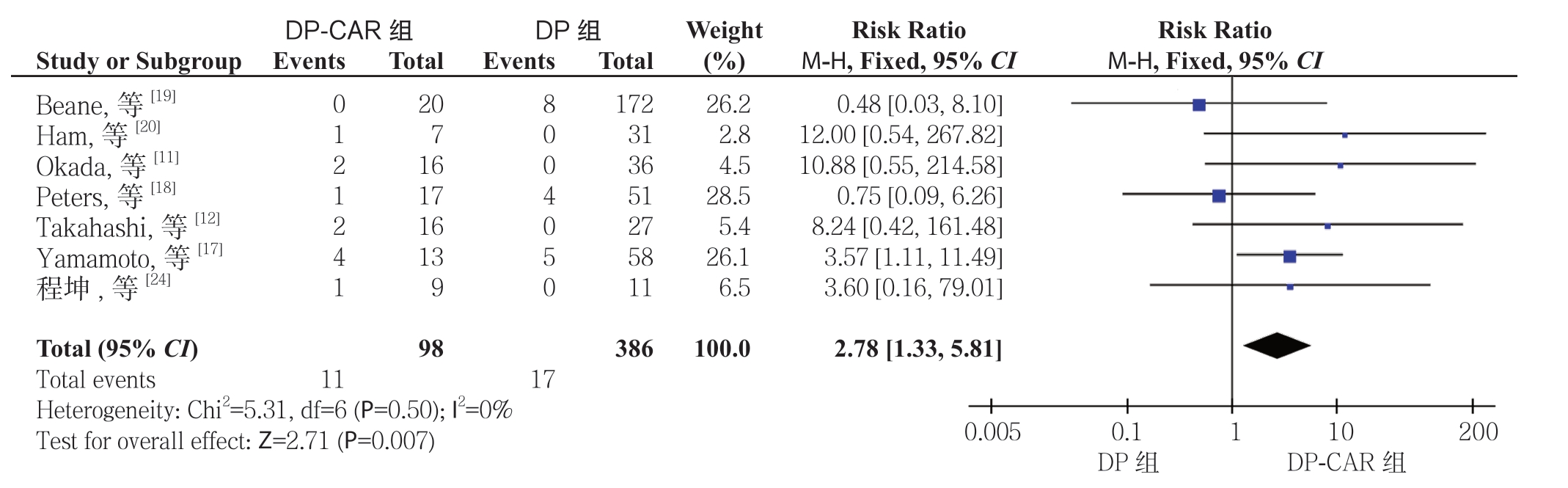
图6 DP-CAR 组与DP 组术后胃排空延迟发生率比较
Figure 6 Comparison of the incidence rates of postoperative delayed gastric emptying between DP-CAR group and DP group
2.2.5 再次手术率 4 项研究[12, 19, 21, 24]报道了再次手术率,DP-CAR 组为11.1%(8/72),而DP组为0.9%(3/343)。Meta 分析结果显示两组术后再次手术率有统计学差异,且DP-CAR 组的再次手术率明显高于DP 组(RR=10.96,95% CI=3.27~36.74,P=0.000 1)(图7)。
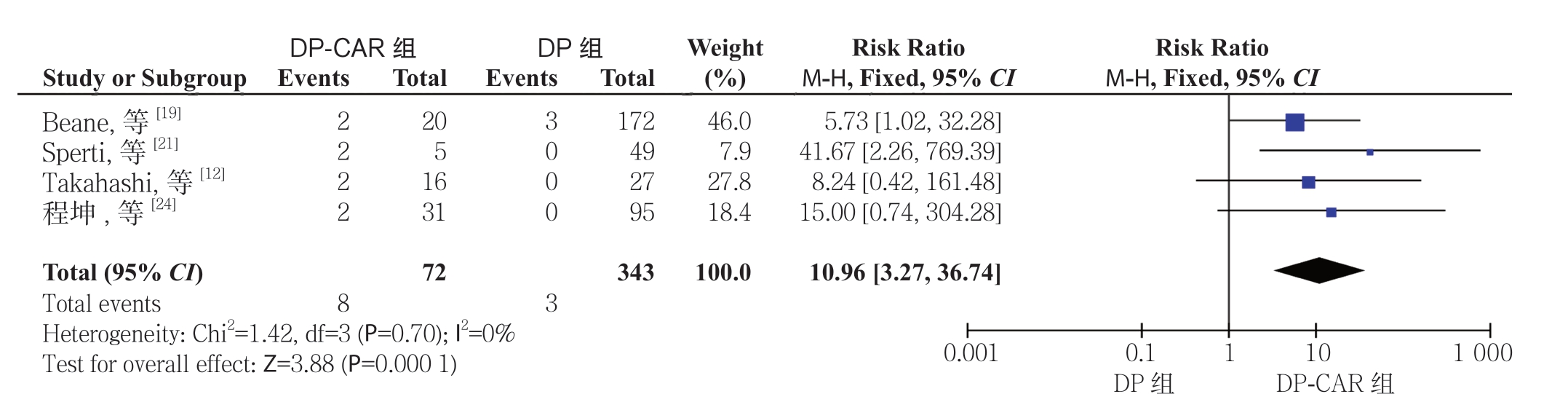
图7 DP-CAR 组与DP 组术后再次手术率比较
Figure 7 Comparison of the reoperation rates between DP-CAR group and DP group
2.2.6 手术时间和失血量 13 项研究[8-9, 11-12, 17-25]结果显示,DP-CAR 组的手术时间比DP 组延长约92 min(MD=91.98,95% CI=60.63~123.34,P<0.000 01),且差异具有统计学意义。此外,10 篇研究[8-9, 11-12, 17-18, 22-25]的Meta 分析结果显示,DP-CAR 组术中失血量明显多于DP 组(MD=275.33,95% CI=135.95~414.71,P=0.000 1),差异具有统计学意义。
2.3 发表偏倚及敏感度分析
本研究中纳入的文献主要是中国、日本的研究,欧美国家的研究相对较少。根据胰瘘发生率绘制漏斗图(图8),各研究均分布在漏斗的上部,但对称性较差,故本研究存在一定的发表偏倚,应用去单项研究法来进行敏感度分析,依次剔除1篇文献后观察合并值的变化情况,结果显示合并值无明显变化,故本研究所得结果具有较好可靠性及稳定性。
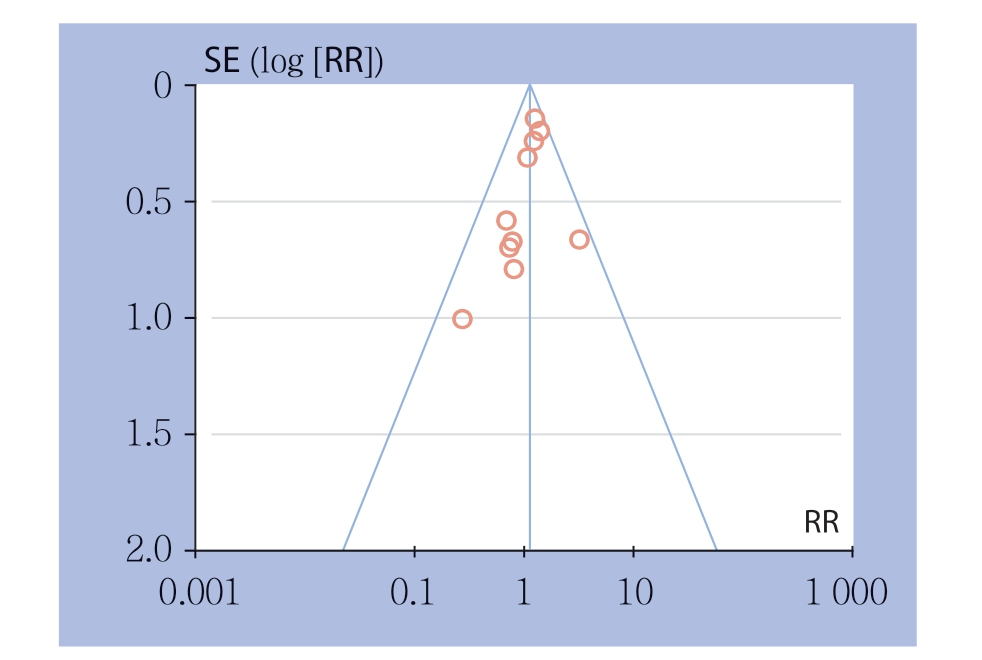
图8 DP-CAR 组与DP 组胰瘘发生率的漏斗图
Figure 8 Funnel plot of incidence of postoperative pancreatic fistula of DP-CAR group and DP group
3 讨 论
DP-CAR在局部进展期胰体尾癌治疗中受到越来越多外科医生的重视。本Meta分析的结果显示:与DP组相比,DP-CAR组在术后近期病死率、再次手术率、临床相关性胰瘘、胃排空延迟的发生率、手术时间及失血量均较高,而其R 0切除率仍较低,但其在术后1、2、3年生存率、术后并发症总发生率及胰瘘的发生率等方面,DP-CAR组与DP组无统计学差异。
R 0切除率及术后生存期是评价手术效果的重要指标。本Meta分析结果显示,DP-CAR组的R0切除率合并值为62.7%,其低于DP组;但其术后1、2、3年总生存率与DP组无统计学差异。大部分胰体尾癌患者在诊断时就失去了手术机会,但随着DP-CAR术式在局部进展期胰体尾中的应用,为CA及其分支受累的局部进展期胰体尾癌患者带来了手术机会[14],在本Meta分析中尽管DP-CAR组R0切除率仍低于DP组,但其可达62.7%。而与DP组相比,DP-CAR组R 0切除率较低原因有:首先,行DP-CAR的患者术前因肿瘤已侵犯CA、CHA等重要血管既往被定义是不可切除性肿瘤;其次,与DP组相比,DP-CAR组的患者往往肿瘤更大、病理及临床分期更晚,淋巴结阳性率更高[8-9, 11-12, 17, 20]。尽管R 0切除率仍低于DP组,但其术后生存获益与DP组无显著性差异,且Yamamoto及Hyemin等[17, 19]的研究结果显示,DP-CAR组患者的术后中位生存期及生存率明显高于保守治疗组的患者。
有9项研究[8-9, 12, 17-19, 21-22, 25]报道了术后并发症总发生率,D P-C A R 及D P 组总发生率分别为56.5%(111/196)、46.9%(408/870),但Meta分析结果显示两者差异无统计学意义(RR=1.23,95% CI=0.97~1.55,P=0.09)。关于术后近期病死率和再次手术率,此前发表的1篇Meta分析表明两者无统计学差异[32],但本Meta分析结果显示:DP-CAR组术后近期病死率及再次手术率显著高于DP组。DP-CAR组患者术后近期死亡最常见的原因是胰瘘及腹腔出血[8, 12],而导致再次手术最常见的原因是腹腔出血[21, 25]。
胰瘘是胰腺术后最常见的并发症之一。本Meta分析结果表明,DP-CAR组术后胰瘘发生率为36.1%,但与DP组相比无统计学差异,与先前发表的Meta分析[32]的结果一致。在本研究中,对胰瘘进一步进行了分级分析,结果显示临床相关性胰瘘(B级、C级)的发生率DP-CAR组显著高于DP组。Yamamoto等[8]报道DP-CAR组临床相关胰瘘发生率高与胰腺实质斜切面积较大有关;而侍力刚等[25]认为术中CA切除后,残余胰头组织血供受到影响,动脉灌注减少,进而影响胰腺残端组织愈合。
缺血性并发症是DP-CAR术后的特异性并发症,包括胃缺血、肝缺血、十二指肠缺血等。DPCAR术后胃缺血性并发症最为常见,主要表现术后胃排空延迟,本Meta分析结果显示,DP-CAR术后胃排空延迟的发生率为11.2%,显著高于DP组。Sato和Okada等[37-38]报道,DP-CAR术中保留或重建胃左动脉是一种安全可行的方法,可以明显降低胃缺血及胃排空延迟等并发症的风险。DPCAR术后肝缺血是比较常见的并发症,主要表现为术后转氨酶一过性升高,经治疗后大部分患者在1~2周内就可恢复至正常[12,17,24-25],而严重的肝缺血性并发症很少发生,在本研究纳入的237例患者中,仅2例发生了局灶性肝梗死[12,17]。在本Meta分析纳入的研究中,Okada等[11]报道了1例DP-CAR术后1周十二指肠发生缺血穿孔的病例,但其在内镜下治疗成功,且患者预后良好。减少DP-CAR术后缺血性并发症的关键之一是术中评估CA切除后是否需要动脉重建,目前最常用的方法是术中夹闭CA后,通过触摸肝固有动脉搏动的强弱程度来判断。此外,有研究[39-41]报道可通过测量第3肝段动脉血流速度、肝静脉血氧饱和度、肝动脉压的变化来判断是否需要重建动脉血管。另一方面,Hirano及Kondo等[26, 42]报道术前栓塞CHA可增加肝动脉的侧支血流,进而减少DP-CAR术后肝、胆囊缺血等并发症。故术前行CHA栓塞,术中保留或重建胃左动脉可能进一步减少缺血性并发症的发生。
手术时间和失血量是外科手术的重要评价指标。本Meta结果显示,与DP组相比,DP-CAR组患者的手术时间明显延长,术中失血量显著增多,这与Go ng 等[32]的Meta 分析结论一致。而导致DP-CAR手术时间长,出血量多的原因有:首先,DP-CAR手术复杂,大部分研究中心该术式开展DP-CAR例数<20例,术者的手术经验相对较少[9, 11-12, 17-24];其次,行DP-CAR的患者其肿瘤侵犯了CA及CHA等重要血管,粘连较严重,分离较困难,且术中需行CA切除,进一步增加了手术的难度和出血的风险;最后,DP-CAR的患者往往侵犯胃、结肠等周围脏器,术中需要联合多器官切除,而最常见的周围脏器切除是胃、结肠、小肠、左肾和肾上腺等[12, 20-22]。上述因素均可导致DP-CAR组手术时间的延长及失血量的增加。
本文纳入了更全面的研究,增加了样本量,并采用系统严谨的方法处理分析数据,提高本Meta分析的质量。但该研究仍存在一些局限性:⑴ 本篇Meta分析纳入的13篇研究,大部分为单中心、回顾性NRCS,仅有2篇为多中心研究,1篇为前瞻性研究;⑵ 纳入本Meta分析的研究样本量均较小,部分研究的DP-CAR组的病例数<10;⑶ 纳入的研究超过一半的研究时间>10年,导致各研究间异质性较大;⑷ 目前胰腺癌治疗模式较前有明显的改变,而大量的研究表明新辅助治疗的应用可提高局部进展期胰体尾癌的R 0切除率,延长患者的总生存期,但本Meta分析未进行相关研究;⑸ 本研究纳入的文献主要是中国、日本的研究,欧美国家的研究相对较少,存在一定发表偏倚。因此本文所得结果具有一定的局限性,需要高质量、大样本、多种治疗模式结合的研究来进一步评价DPCAR在局部进展期胰腺癌中的临床疗效及安全性。
DP-CAR对于局部进展期胰体尾癌患者没有明显的生存获益,且其术后近期病死率、再次手术率,临床相关性胰瘘和胃排空延迟的发生率均明显高于DP,故应慎重选择该术式。
[1] Plauchu M, Chapuy H.Pancreatic cancer[J].Rev Infirm Assist Soc,1969, 19(5):485-489.
[2] Hartwig W, Werner J1, Jäger D, et al.Improvement of surgical results for pancreatic cancer[J].Lancet Oncol, 2013, 14(11):e476-485.doi: 10.1016/S1470-2045(13)70172-4.
[3] Varadhachary GR, Tamm EP, Abbruzzese JL, et al.Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy[J].Ann Surg Oncol, 2006, 13(8):1035-1046.doi: 10.1245/ASO.2006.08.011.
[4] Johnson CD, Schwall G, Flechtenmacher J, et al.Resection for adenocarcinoma of the body and tail of the pancreas[J].Br J Surg,1993, 80(9):1177-1179.doi: 10.1002/bjs.1800800937.
[5] Jimenez RE, Warshaw AL, Rattner DW, et al.Impact of laparoscopic staging in the treatment of pancreatic cancer[J].Arch Surg, 2000, 135(4):409-414.doi: 10.1001/archsurg.135.4.409.
[6] Shoup M, Conlon KC, Klimstra D, et al.Is extended resection for adenocarcinoma of the body or tail of the pancreas justified?[J]J Gastrointest Surg, 2003, 7(8):946-952.doi: 10.1016/j.gassur.2003.08.004.
[7] Alexakis N, Halloran C, Raraty M, et al.Current standards of surgery for pancreatic cancer[J].Br J Surg, 2004, 91(11):1410-1427.doi: 10.1002/bjs.4794.
[8] Yamamoto T, Satoi S, Kawai M, et al.Is distal pancreatectomy with en-bloc celiac axis resection effective for patients with locally advanced pancreatic ductal adenocarcinoma? -Multicenter surgical group study[J].Pancreatology, 2018, 18(1):106-113.doi: 10.1016/j.pan.2017.11.005.
[9] Sugiura T, Okamura Y, Ito T, et al.Surgical Indications of Distal Pancreatectomy with Celiac Axis Resection for Pancreatic Body/Tail Cancer[J].World J Surg, 2017, 41(1):258-266.doi: 10.1007/s00268-016-3670-3.
[10] Barugola G, Partelli S, Marcucci S, et al.Resectable pancreatic cancer: who really benefits from resection?[J].Ann Surg Oncol,2009, 16(12):3316-3322.doi: 10.1245/s10434-009-0670-7.
[11] Okada KI, Kawai M, Tani M, et al.Surgical strategy for patients with pancreatic body/tail carcinoma: Who should undergo distal pancreatectomy with en-bloc celiac axis resection?[J].Surgery,2013, 153(3):365-372.doi: 10.1016/j.surg.2012.07.036.
[12] Takahashi Y, Kaneoka Y, Maeda A, et al.Distal pancreatectomy with celiac axis resection for carcinoma of the body and tail of the pancreas[J].World J Surg, 2011, 35(11):2535-2542.doi: 10.1007/s00268-011-1245-x.
[13] Appleby LH.The coeliac axis in the expansion of the operation for gastric[J].Cancer, 1953, 6(4):704-707.
[14] Nimura Y, Hathora T, Miura K, et al.A case of advanced carcinoma of the body and tail of the pancreas resected by the Appleby operation (in Japanese)[J].Operation, 1976, 30:885-889.
[15] Kondo S, Katoh H, Hirano S, et al.Results of radical distal pancreatectomy with en bloc resection of the celiac artery for locally advanced cancer of the pancreatic body[J].Langenbecks Arch Surg, 2003, 388(2):101-106.doi: 10.1007/s00423-003-0375-5.
[16] Nakao A, Takeda S, Inoue S, et al.Indications and techniques of extended resection for pancreatic cancer[J].World J Surg, 2006,30(6):976-982.doi: 10.1007/s00268-005-0438-6.
[17] Yamamoto Y, Sakamoto Y, Ban D, et al.Is celiac axis resection justified for T4 pancreatic body cancer?[J].Surgery, 2012,151(1):61-69.doi: 10.1016/j.surg.2011.06.030.
[18] Peters NA, Javed AA, Cameron JL, et al.Modified Appleby Procedure for Pancreatic Adenocarcinoma: Does Improved Neoadjuvant Therapy Warrant Such an Aggressive Approach?[J].Ann Surg Oncol, 2016,23(11):3757-3764.doi: 10.1245/s10434-016-5303-3.
[19] Beane JD, House MG, Pitt SC, et al.Distal pancreatectomy with celiac axis resection: what are the added risks?[J].HPB (Oxford),2015, 17(9):777-784.doi: 10.1111/hpb.12453.
[20] Ham H, Kim SG, Kwon HJ, et al.Distal pancreatectomy with celiac axis resection for pancreatic body and tail cancer invading celiac axis[J].Ann Surg Treat Res, 2015, 89(4):167-175.doi: 10.4174/astr.2015.89.4.167.
[21] Sperti C, Berselli M, Pedrazzoli S.Distal pancreatectomy for bodytail pancreatic cancer: is there a role for celiac axis resection?[J].Pancreatology, 2010, 10(4):491-498.doi: 10.1159/000276984.
[22] Mayumi T, Nimura Y, Kamiya J, et al.Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas [J].Int J Pancreatol, 1997, 22(1):15-21.doi:10.1007/BF02803900.
[23] Wu X, Tao R, Lei R, et al.Distal pancreatectomy combined with celiac axis resection in treatment of carcinoma of the body/tail of the pancreas: a single-center experience[J].Ann Surg Oncol, 2010,17(5):1359-1366.doi: 10.1245/s10434-009-0840-7.
[24] 程坤, 韩玮, 林海, 等.改良Appleby手术治疗局部进展期胰体尾癌安全性评价[J].新疆医学, 2017, 47(1):5-10.Cheng K, Han W, Lin H, et al.Safety evaluation of modified Appleby procedure in the treatment of locally advanced pancreatic body and tail cancer[J].Xinjiang Medical Journal, 2017, 47(1):5-10.
[25] 侍力刚, 冀蒙, 陈军, 等.联合腹腔干切除胰体尾癌根治术疗效及安全性分析[J].中国实用外科杂志, 2017, 37(7):784-787.doi:10.19538/j.cjps.issn1005-2208.2017.07.21.Shi LG, Ji M, Chen J, et al.Clinical efficacy and safety of distal pancreatectomy with celiac axis resection for pancreatic body/tail cancer[J].Chinese Journal of Practical Surgery, 2017, 37(7):784-787.doi:10.19538/j.cjps.issn1005-2208.2017.07.21.
[26] Hirano S, Kondo S, Hara T, et al.Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results[J].Ann Surg, 2007, 246(1):46-51.doi:10.1097/01.sla.0000258608.52615.5a.
[27] Hishinuma S, Ogata Y, Tomikawa M, et al.Stomach-preserving distal pancreatectomy with combined resection of the celiac artery:radical procedure for locally advanced cancer of the pancreatic body[J].J Gastrointest Surg, 2007, 11(6):743-749.doi: 10.1007/s11605-007-0143-x.
[28] Denecke T, Andreou A, Podrabsky P, et al.Distal pancreatectomy with en bloc resection of the celiac trunk for extended pancreatic tumor disease: an interdisciplinary approach[J].Cardiovasc Intervent Radiol, 2011, 34(5):1058-1064.doi: 10.1007/s00270-010-9997-5.
[29] Ge L, Tian JH, Li YN, et al.Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study[J].J Clin Epidemiol, 2018, 93:45-55.doi: 10.1016/j.jclinepi.2017.10.012.
[30] Tian J, Zhang J, Ge L, et al.The methodological and reporting quality of systematic reviews from China and the USA are similar[J].J Clin Epidemiol, 2017, 85:50-58.doi: 10.1016/j.jclinepi.2016.12.004.
[31] Yao L, Sun R, Chen YL, et al.The quality of evidence in Chinese meta-analyses need to be Improved[J].J Clin Epidemiol, 2016,74:73-79.doi: 10.1016/j.jclinepi.2016.01.003.
[32] Gong H, Ma R, Gong J, et al.Distal Pancreatectomy With En Bloc Celiac Axis Resection for Locally Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis[J].Medicine (Baltimore),2016, 95(10):e3061.doi: 10.1097/MD.0000000000003061.
[33] Stang A.Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in metaanalyses[J].Eur J Epidemiol, 2010, 25(9):603-605.doi: 10.1007/s10654-010-9491-z.
[34] Qiu J, Yuan H, Chen S, et al.Single-port versus conventional multiport laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials and nonrandomized studies[J].J Laparoendosc Adv Surg Tech A, 2013, 23(10):815-831.doi:10.1089/lap.2013.0040.
[35] Higgins JP, Thompson SG.Quantifying heterogeneity in a metaanalysis [J].Stat Med, 2002, 21(11):1539-1558.doi: 10.1002/sim.1186.
[36] Bassi C, Dervenis C, Butturini G, et al.Postoperative pancreatic fistula: an international study group (ISGPF) definition[J].Surgery,2005, 138(1):8-13.doi: 10.1016/j.surg.2005.05.001.
[37] Sato T, Saiura A, Inoue Y, et al.Distal Pancreatectomy with En Bloc Resection of the Celiac Axis with Preservation or Reconstruction of the Left Gastric Artery in Patients with Pancreatic Body Cancer[J].World J Surg, 2016, 40(9):2245-2253.doi: 10.1007/s00268-016-3550-x.
[38] Okada KI, Kawai M, Tani M, et al.Preservation of the left gastric artery on the basis of anatomical features in patients undergoing distal pancreatectomy with celiac axis en-bloc resection (DP-CAR)[J].World J Surg, 2014, 38(11):2980-2985.doi: 10.1007/s00268-014-2702-0.
[39] Hirai I, Kimura W, Kamiga M, et al.The significance of intraoperative Doppler ultrasonography in evaluating hepatic arterial flow when assessing the indications for the Appleby procedure for pancreatic body cancer[J].J Hepatobiliary Pancreat Surg, 2005, 12(1):55-60.doi: 10.1007/s00534-004-0939-y.
[40] Miyakawa S, Horiguchi A, Hanai T, et al.Monitoring hepatic venous hemoglobin oxygen saturation during Appleby operation for pancreatic cancer[J].Hepatogastroenterology, 2002, 49(45):817-821.
[41] Mittal A, de Reuver PR, Shanbhag S, et al.Distal pancreatectomy,splenectomy, and celiac axis resection (DPS-CAR): common hepatic arterial stump pressure should determine the need for arterial reconstruction[J].Surgery, 2015, 157(4):811-817.doi:10.1016/j.surg.2014.10.006.
[42] Kondo S, Katoh H, Shimizu T, et al.Preoperative embolization of the common hepatic artery in preparation for radical pancreatectomy for pancreas body cancer[J].Hepatogastroenterology, 2000,47(35):1447-1449.
