过敏性紫癜(henoch-schonlein purpura,HSP)是一种较常见的毛细血管变态反应性疾病,临床表现为血液逸于皮肤、黏膜之下,出现瘀点瘀斑,可伴关节肿痛、腹痛、便血、血尿和蛋白尿[1-2]。腹型HSP主要累及消化道,以腹痛、恶心呕吐、便血等特点起病,因临床特征不典型,一般较易误诊为消化性溃疡、急性阑尾炎、急性胃肠炎等疾病[3]。本文回顾性分析中南大学湘雅医院消化内科收治的45例腹型HSP住院患者的临床资料,分析总结其临床表现、实验室指标、内镜、病理特点及治疗和预后,旨在加深对该病的认识,提高诊治水平,避免误诊。
1 资料与方法
1.1 一般资料
2012年1月—2018年1月在中南大学湘雅医院住院期间,年龄≥14岁,以腹痛为主要表现的HSP患者45例;所有患者血小板计数≥100×109/L及凝血功能正常,同时排除其他原因引起的血管炎及紫癜[4]。
1.2 研究方法
查阅住院病历,对45例患者的一般资料、临床表现、实验室数据、胃肠镜、小肠镜、影像学资料、病理结果、治疗方案及预后进行分析。
2 结 果
2.1 发病年龄与年龄构成
4 5 例 腹 型H S P 患 者 中 男3 4 例,女1 1 例,男女比例3.1:1,年龄1 4 ~6 8 岁,平均发病年龄(31.1±18.6)岁,其中≤18岁16例,19~30岁12例,31~60岁10例,>60岁7例,以18岁以下的青少年居多。
2.2 发病时间
45例腹型HSP患者中,春季起病6例,夏季起病9例,秋季起病15例,冬季起病15例,以秋冬起病多见。
2.3 临床表现
首发症状,所有患者中首发症状有:腹痛(24例,53.3%),皮疹(14例,31.1%),腹痛伴皮疹(5例,11.1%),发热(1例,2.2%),腹泻(1例,2.2%)。其他伴随症状:恶心(10例, 22.2%)、呕吐(13例,28.9%)、便血(10例,2 2.2%)、腹泻(9 例,2 0.0%),双侧膝关节疼痛(6 例,1 3.3%)、双下肢水肿(1 例,2.2%)、反酸(1 例,2.2%)、腹胀(1 例,2.2%)。腹痛部位及其性质:4 0 例伴有腹痛的HSP患者中,脐周疼痛(6例,15.0%)、中上腹部疼痛(17 例,4 2.5%)、下腹部疼痛(7例,17.5%)、剑突下疼痛(2例,5.0%)、全腹部疼痛(3例,7.5%)、上腹部—右下腹部转移性疼痛(1例,2.5%)、位置描述不明(6例,15.0%);其中,阵发性钝痛(1 1 例,2 7.5%)、阵发性绞痛(1 0 例,2 5.0%)、持续性隐痛(1 0 例,25.0%)、持续性刺痛(3例,7.5%)、描述不详细(5例,12.5%);此外,28例有腹部压痛,17例无明显腹部阳性体征。皮疹特征:所有患者中,出现皮疹(34例,75.6%),大部分为对称性分布的出血性皮疹(压之不褪色),多分布于双下肢及臀部。
2.4 实验室检查
常规生化: 血常规, 白细胞计数升高3 2 例(7 1.1%),其中中性粒细胞升高3 8 例(8 4.4%)、淋巴细胞降低1 1 例(2 4.4%),血红蛋白降低1 0 例(2 2.2%),血小板升高1 2 例(2 6.7%)。尿常规:尿蛋白阳性1 3 例(28.9%),尿潜血阳性14例(31.1%),尿红细胞阳性8例(17.8%)、尿酮体阳性15例(33.3%)、尿胆原阳性4 例(9.0%)。大便常规+隐血:大便隐血试验阳性3 3 例(7 3.3%)、弱阳性3 例(6.7%)、阴性9 例(2 0%)。血白蛋白降低 35例(77.8%)、血沉升高14例(35.0%)、C反应蛋白升高(CRP)27例(60.0%)、降钙素原升高 12例(26.7%)。免疫学指标:所有HSP患者中 25例行免疫全套检查,其中IgA升高4例(16%)、补体C3或C4降低6例(24.0%);此外,12例行抗核抗体谱测定,2 例抗核抗体阳性,滴度均为1:4 0,6 例行病毒检查,其中1 例合并E B 病毒感染,2 4 例行抗O 抗体、类风湿因子检测,3 例抗O 抗体阳性,类风湿因子均正常,1 1 例行狼疮检查和5 例行血管炎检查,均未发现异常。食物不耐受1 4 项:3 2 例患者行食物不耐受检查,其中1 9 例含有食物过敏原(5 9.4%),蛋清蛋黄 11例(57.9%)、大豆5例(26.3%)、西红柿、大米、牛奶、鳕鱼3例(15.8%)、玉米、蟹2例(10.5%)、花生、小麦1例(5.3%)。
2.5 内镜检查
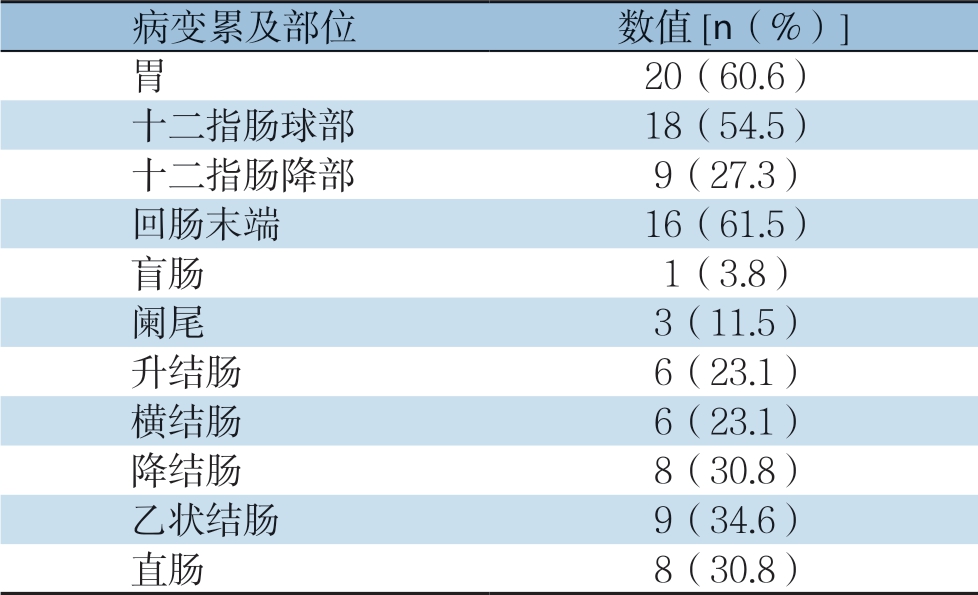
所有H S P 患者中有3 3 例接受胃镜检查,主要镜下表现为胃、十二指肠黏膜的充血水肿及片状糜烂、溃疡的形成,主要累及部位分别为十二指肠球部1 8 例(5 4.5%)、胃窦1 0 例(3 0.3%)、十二指肠降部9 例(2 7.3%)、胃体4例(12.1%)、胃底2例(6.1%)、胃角2例(6.1%)、胃大弯2例(6.1%)。26例患者接受肠镜检查,主要表现为肠黏膜的充血水肿,大部分可见点片状红斑及糜烂,少部分可见多发溃疡和肠黏膜渗血(图1),主要累及部位,回肠末端16例(61.5%)、乙状结肠9例(34.6%)、降结肠8例(30.8%)、直肠8例(30.8%)、升结肠6例(23.1%)、横结肠6例(23.1%)、阑尾 3例(11.5%)、盲肠1例(3.8%),4例未见明显异常(表1)。3例行小肠镜检查,主要表现为回肠中下段黏膜充血肿胀,绒毛短粗,可见多发黏膜,糜烂和不规则白苔溃疡,表浅溃疡为主。
表1 腹型HSP 患者胃肠道病变累及部位
Table 1 The involved areas of the gastrointestinal tract of the abdominal HSP patients
2.6 影像学、病理检查
45例患者中有7例行腹部CT检查,均表现为肠壁增厚,其中2 例伴有腹膜后、肠系膜淋巴结肿大。45例患者中有12例行病理活检,主要病理表现为黏膜慢性炎症,部分区域可见嗜酸性粒细胞,淋巴细胞及浆细胞浸润。
2.7 治疗及预后
所有患者诊断明确后给予综合治疗,包括避免接触过敏原、抑酸护胃、解痉止痛、改善血管通透性、补液等对症处理。所有患者中有40例(88.9%)给予糖皮质激素(甲泼尼龙或地塞米松)治疗,腹痛、皮疹明显缓解,均未出现并发症,于2~3周内治愈。45例患者中有6例复发,复发率为13.3%,复发时间1个月至2年不等,予以调整激素用量及对症治疗后治愈。
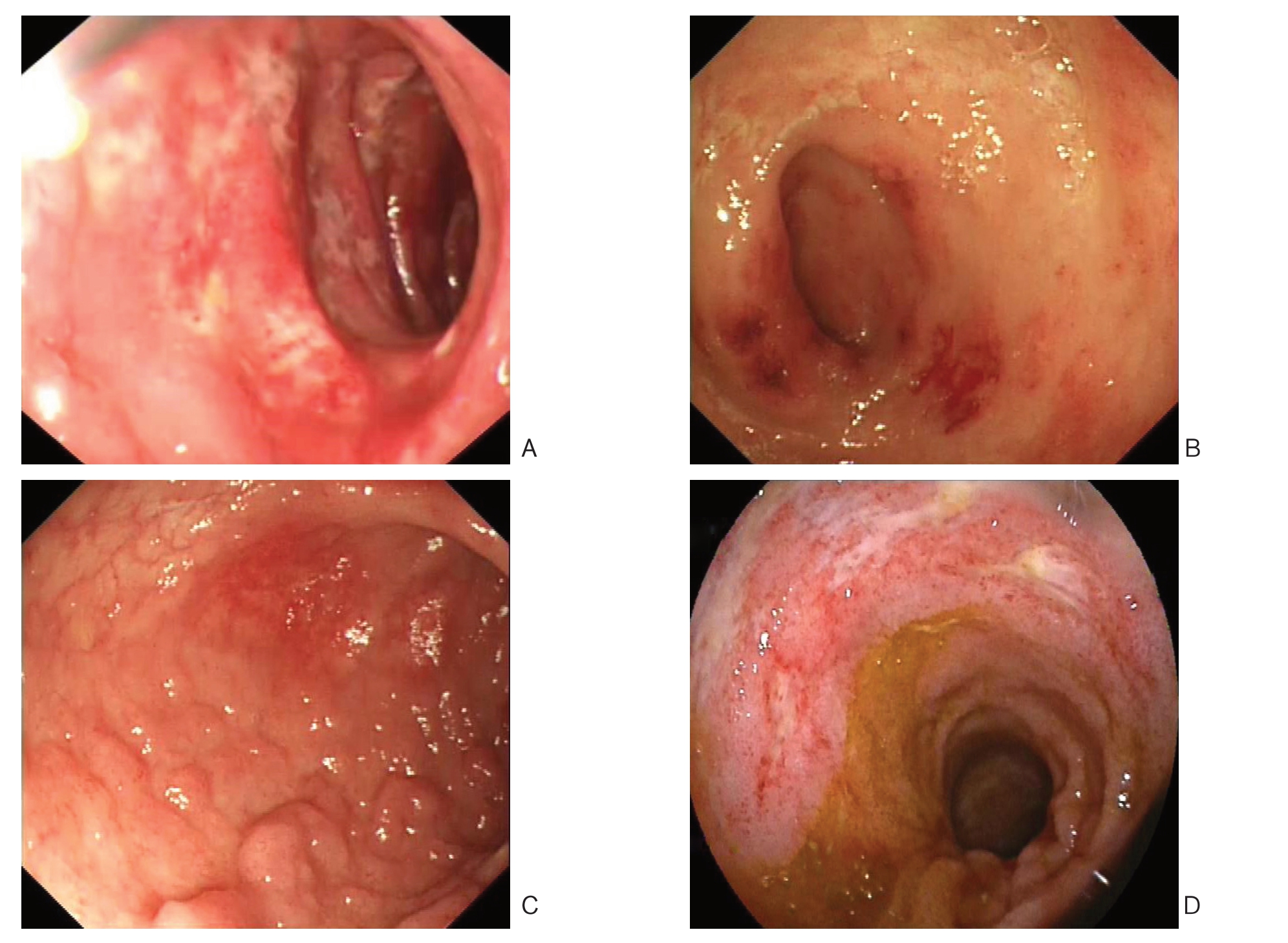
图1 腹型HSP 患者胃肠镜表现 A:十二指肠球降部充血糜烂;B:回肠末端充血水肿;C:回肠末端散在结节样改变; D:回肠中下段多发浅表溃疡
Figure 1 Gastrointestinal endoscopic findings in abdominal HSP patients A: Mucosal congestion and erosion in the duodenal bulb; B: Mucosal congestion and edema in the distal ileum; C: Sporadic nodular lesions in the distal ileum; D: Multiple shallow ulcers in the middle and lower segments of ileum
3 讨 论
HSP是一种全身小血管炎,主要累及毛细血管、小静脉或小动脉,是最常见的免疫复合物介导的血管炎之一,多见于儿童和青少年,发病率约6.1/100 000~20.4/100 000不等[5-8],其病变主要累及皮肤、关节、胃肠道、肾脏的小血管,引起皮肤紫癜、腹痛、便血、关节疼痛、蛋白尿、血尿等相关症状[1-2, 9]。
目前,HSP的病因及发病机制尚未明确,可能与以下几种因素有关:⑴ 遗传易感性,虽然大多数HSP患者为散发病例,但有相关研究[10-14]报道HSP具有家族聚集性。HSP是一种免疫复合物介导的疾病,基于主要组织相容性基因可能参与该疾病的发病机制这一假设,越来越多的研究关注人类白细胞抗原(HLA)与HSP之间的联系。HLADRB1多态性与土耳其儿童的HSP患病风险增加有关,同时HLA-DRB1等位基因与HSP易感性和病情严重程度相关[15]。Amoli等[16-17]报道HLA-B35、HLADQA1是HSP患者发生肾损害的危险等位基因。此外,血管紧张素原基因对HSP表达和结果的影响已经被研究,血管紧张素原基因在无肾损害的HSP患者和有肾损害的HSP患者之间的基因型分布差异与HSP患者肾损伤的发生有显著的相关性[18]。另外,有研究[9, 19]表明MEFV基因突变是HSP发生的危险因素,与诊断年龄、关节炎发生频率、CRP水平升高等临床表现存在相关性。⑵ 由于HSP多在秋冬季节细菌或病毒感染后出现,由此推测感染在HSP的病因学中起一定作用。微生物拥有类似于血管壁的抗原结构,从而诱导产生交叉反应的自身抗体,其中溶血性链球菌、EB病毒、柯萨奇病毒、肺炎支原体均可能作为诱发因素,导致机体发生变态反应,从而触发HSP的发生[20-23]。此外,幽门螺杆菌(HP)的一种毒力因子被证明可以促进半乳糖缺乏的IgA1的产生,与未感染的儿童相比,HP阳性儿童发生HSP的几率高3.8倍[20,23-25]。 ⑶免疫异常,HSP是一种由免疫复合物介导的小血管炎,以免疫球蛋白A1(IgA1)沉积、补体因子和中性粒细胞浸润为特征。许多血清学研究显示,50%的HSP患者在急性期血清IgA水平升高,在HSP患者急性期也发现循环中含有IgA的免疫复合物的冷球蛋白[26]。IgA是人类免疫球蛋白家族中的一员,其有两种亚型,分别为IgA1和IgA2。IgA1是主要的亚型,占血清IgA的80%,IgA1在重链上有一个铰链区域,有5~6个O-聚糖位点,其末端糖基更容易被自身抗体识别,容易引起IgA聚集,从而形成大分子复合物,引起广泛的毛细血管炎,造成血管壁通透性和脆性增高,导致皮下组织、黏膜及内脏器官出血及水肿;另外,细胞因子、补体、内皮损伤、自身抗体在HSP的发病机制中也发挥重要作用[27-28]。
HSP可累及全身多个系统,根据相关体征及临床症状可将其分为皮肤型、腹型、肾型、关节型,若有两种以上并存时称为混合型。据统计,5 1%~7 4%的H S P 患者可伴有消化道症状,本文中,以腹痛为首发症状起病的患者为2 4 例,约53.3%,其他伴随症状有恶心呕吐、便血、黑便、腹胀、腹泻、反酸,以脐周及中上腹的阵发性钝痛为主,症状及体征均不典型,对患者的早期诊治造成了一定困难,且易被误诊,一般常被误诊为消化性溃疡、急性胃肠炎、急性胃黏膜病变、缺血性肠病等疾病,更有甚者误诊为“急腹症”行外科手术探查。
H S P 患者的实验室检查也无相关特征性指标,在本组资料中大部分患者有中性粒细胞升高、CRP升高以及白蛋白降低,这些指标均无明显特异性,对HSP的早期诊治增加了难度。目前已发现多种与HSP相关的IgA自身抗体,包括IgA类风湿因子、IgA抗心磷脂抗体、IgA抗内皮细胞抗 体[22-23,29]。虽然上述部分IgA自身抗体可能在HSP中发挥致病作用,但是否能够成为诊断HSP的生物标志物还需要更多的研究来验证。
在早期出现胃肠道症状的HSP患者中,胃肠镜检查是非常必要的。本组资料中大部分患者接受了胃肠镜检查,胃肠道的内镜下表现主要为胃肠道黏膜的大片红斑形成、水肿、糜烂及溃疡,内镜下的不同表现与机体炎症反应的严重程度相关。本组HSP患者中有33例行胃镜检查,主要镜下表现为胃、十二指肠黏膜的充血水肿及片状糜烂,少数伴溃疡的形成,病变之间黏膜正常,主要累及部位分别为十二指肠球部及降部、胃窦等,与其他文献[30-31]报道基本一致。26例患者行肠镜检查,主要累及回肠末端,除黏膜充血水肿外,小部分可见多形性、不规则溃疡,且溃疡发生率较上消化道高。据报道,严重腹痛、持续性紫癜或血浆XIII因子活性降低的HSP患者发生肾损伤的概率较高,因此,在胃肠道症状先于皮肤症状出现的情况下,早期诊断HSP非常重要。当胃肠镜均无阳性发现,对于消化道症状较重患者,可进一步完善小肠检查,本组3例患者接受了小肠镜检查,主要表现为回肠中下段黏膜充血肿胀,绒毛短粗,可见多发黏膜糜烂和不规则白苔溃疡,以表浅溃疡为主。另据相关报道[32],胶囊内镜在评估腹型HSP患者胃肠道累及情况时,有一定的诊断价值,且具有更好的耐受性,同时,由于肠壁广泛水肿,注意警惕胶囊内镜滞留。在临床中,考虑经济性,胃肠镜常作为首选检查手段,对于以腹痛为首发症状,胃肠道广泛充血水肿,应考虑HSP。
此外,有7例患者行腹部CT检查,主要表现为节段性肠管损伤,肠管管壁水肿增厚,同时伴受累部位周围淋巴结肿大,少数可出现腹腔积液,由于肠壁水肿无明显特异性,运用CT对HSP进行诊断时,应结合患者症状体征综合考虑,注意与肠炎等疾病相鉴别。HSP的组织病理学以白细胞血管炎、IgA免疫复合物沉积为特征[33-34]。肠道活检显示,64%的患者存在颗粒状IgA沉积,而其他各种胃肠道疾病的这一比例仅为10%[35]。胃肠道组织学主要表现为白细胞破碎性血管炎、血管周围中性粒细胞浸润、内皮细胞肿胀,在本组患者中来自胃肠道的活检标本仅显示为非特异性炎症细胞浸润。诊断明确的HSP患者,早期应用糖皮质激素可以使消化道症状和皮疹得到明显缓解,本组患者有效率达到88.9%,如疗效不佳或合并紫癜性肾炎,可联合使用免疫抑制剂。另外,丙种球蛋白被证明对皮质类固醇不耐受的个体和在皮质类固醇逐渐减少时多次复发的个体有效[36-37]。但是,皮质类固醇对于预防严重并发症或复发的有效性仍存在争议。在本组中,完善HP检查的患者数较少,根除HP有利于减轻患者的消化道症状以及减少复发,对伴有HP感染的患者,建议抗HP治疗。
综上所述,腹型HSP临床表现缺乏特异性,体征不典型。对于以消化道症状(腹痛、腹泻、便血)起病的患者,应仔细询问病史和体格检查,特别注意双下肢和臀部是否伴有隐匿性皮疹;结合胃肠镜检查,注意与胃肠炎、消化性溃疡等常见病相鉴别,避免误诊,必要时可进一步行小肠镜、胶囊内镜等小肠检查,达到早诊早治HSP,避免和减少相关并发症的出现。
[1]陈灏珠, 林果为, 王吉耀. 实用内科学[M]. 北京:人民卫生出版社, 2013:2480-2482. Chen HZ, Lin GW, Wang JY. Practical internal medicine[M]. Beijing: People's Medical Publishing House, 2013:2480-2482.
[2]Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: Diagnostic and therapeutic aspects[J]. Autoimmun Rev, 2015, 14(7):579-585. doi: 10.1016/j.autrev.2015.02.003.
[3]张海华, 袁伟建. 成人腹型过敏性紫癜的临床特征及误诊分析[J]. 中国内镜杂志, 2012, 18(12):1260-1263. Zhang HH, Yuan WJ. Clinical manifestations and misdiagnosis of anaphylactoid purpura abdominalis in adults[J]. China Journal of Endoscopy, 2012, 18(12):1260-1263.
[4]Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PRES endorsed consensus criteria for the classification of childhood vasculitides[J]. Ann Rheum Dis, 2006, 65(7):936-941. doi: 10.1136/ard.2005.046300.
[5]Butbul Aviel Y, Dafna L, Pilar G, et al. Endothelial function in children with a history of henoch schönlein purpura[J]. Pediatr Rheumatol Online J, 2017, 15(1):3. doi: 10.1186/s12969-016-0135-z.
[6]Pillebout E, Verine J. Henoch-Schönlein purpura in the adult[J]. Rev Med Interne, 2014, 35(6):372-381. doi: 10.1016/j.revmed.2013.12.004.
[7]Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): current state of knowledge[J]. Curr Opin Rheumatol, 2013, 25(2):171-178. doi: 10.1097/BOR.0b013e32835d8e2a.
[8]Roberts PF, Waller TA, Brinker TM, et al. Henoch-Schönlein purpura: a review article[J]. South Med J, 2007, 8(100):821-824. doi: 10.1097/SMJ.0b013e3180f62d0f.
[9]Ozçakar ZB, Yalçinkaya F, Cakar N, et al. MEFV mutations modify the clinical presentation of Henoch-Schönlein purpura[J]. J Rheumatol, 2008, 35(12):2427-2429.
[10]Spyridis N, Salapata M, Fessatou S, et al. Simultaneous presentation of Henoch Schönlein purpura in monozygotic twins[J]. Scand J Infect Dis, 2005, 37(9):703-704. doi: 10.1080/00365540510040197.
[11]Motoyama O, Iitaka K. Familial cases of Henoch-Schönlein purpura in eight families[J]. Pediatr Int, 2005, 47(6):612-615. doi: 10.1111/j.1442-200x.2005.02138.x.
[12]Shin JI, Lee JS. Familial clusturing of Henoch-Schönlein purpura or IgA nephropathy: genetic background or environmental triggers?[J]. Pediatr Dermatol, 2008, 25(6):651. doi: 10.1111/j.1525-1470.2008.00797.x.
[13]Park SJ, Suh JS, Lee JH, et al. Advances in our understanding of the pathogenesis of Henoch-Schönlein purpura and the implications for improving its diagnosis[J]. Expert Rev Clin Immunol, 2013, 9(12):1223-1238. doi: 10.1586/1744666X.2013.850028.
[14]Reamy BV, Williams PM, Lindsay TJ, et al. Henoch-Schönlein purpura[J]. Am Fam Physician, 2009, 80(7):697-704.
[15]Soylemezoglu O, Peru H, Gonen S, et al. CTLA-4 +49 A/G genotype and HLA-DRB1 polymorphisms in Turkish patients with Henoch-Schönlein purpura[J]. Pediatr Nephrol, 2008, 23(8):1239-1244. doi: 10.1007/s00467-008-0837-7.
[16]Amoli MM, Thomson W, Hajeer AH, et al. HLA-B35 association with nephritis in Henoch-Schönlein purpura[J]. J Rheumatol, 2002, 29(5):948-949.
[17]Jin DK, Kohsaka T, Koo JW, et al. Complement 4 locus II gene deletion and DQA1*0301 gene:genetic risk factors for IgA nephropathy and Henoch-Schönlein nephritis[J]. Nephron, 1996, 73(3):390-395. doi: 10.1159/000189098.
[18]Zhou TB, Ou C, Qin YH, et al. A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and Henoch-Schönlein purpura nephritis risk in Asian children[J]. Clin Exp Rheumatol, 2012, 30(2):315-316.
[19]Nikibakhsh AA, Houshmand M, Bagheri M, et al. MEFV gene mutations (M694V, V726A, M680I, and A744S) in Iranian children with Henoch-Schönlein purpura[J]. Pneumologia, 2012, 61(2):84-87.
[20]Axon A. Helicobacter pylori and public health[J]. Helicobacter, 2014, 19(1):68-73. doi: 10.1111/hel.12155.
[21]Novak J, Moldoveanu Z, Julian BA, et al. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system[J]. Adv Otorhinolaryngol, 2011, 72:60-63. doi: 10.1159/000324607.
[22]Yang YH, Wang SJ, Chuang YH, et al. The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-α treatment in children with Henoch-Schönlein purpura[J]. Clin Exp Immunol, 2002, 130(2):352-357. doi: 10.1046/j.1365-2249.2002.01964.x.
[23]Saulsbury FT. Heavy and light chain composition of serum IgA and IgA rheumatoid factor in Henoch-Schönlein purpura[J]. Arthritis Rheum, 1992, 35(11):1377-1380. doi: 10.1002/art.1780351121.
[24]Xiong LJ, Tong Y, Wang ZL, et al. Is Helicobacter pylori infection associated with Henoch-Schönlein purpura in Chinese children? a meta-analysis[J]. World J Pediatr, 2012, 8(4):301-308. doi: 10.1007/s12519-012-0373-1.
[25]Yang M, Li FG, Xie XS, et al. CagA, a major virulence factor of Helicobacter pylori, promotes the production and underglycosylation of IgA1 in DAKIKI cells[J]. Biochem Biophys Res Commun, 2014, 444(2):276-281. doi: 10.1016/j.bbrc.2014.01.050.
[26]Davin JC, Ten Berge IJ, Weening JJ. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis?[J]. Kidney Int, 2001, 59(3):823-834. doi: 10.1046/j.1523-1755.2001.059003823.x.
[27]Yoo EM, Morrison SL. IgA: an immune glycoprotein[J]. Clin Immunol, 2005, 116(1):3-10. doi: 10.1016/j.clim.2005.03.010.
[28]Renfrow MB, Mackay CL, Chalmers MJ, et al. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy[J]. Anal Bioanal Chem, 2007, 389(5):1397-1407. doi: 10.1007/s00216-007-1500-z.
[29]Kawakami T, Watabe H, Mizoguchi M, et al. Elevated serum IgA anticardiolipin antibody levels in adult Henoch-Schönlein purpura[J]. Br J Dermatol, 2006, 155(5):983-987. doi: 10.1111/j.1365-2133.2006.07457.x.
[30]Burger D, Wittmann J. Education and imaging. Gastrointestinal: Henoch-Schönlein purpura[J]. Gastroenterol Hepatol, 2009, 24(8):1473. doi: 10.1111/j.1440-1746.2009.05961.x.
[31]韩跃东, 张超, 张国平, 等. 腹型过敏性紫癜患者临床和消化内镜表现分析[J]. 中国麻风皮肤病杂志, 2017, 33(1):26-28. Han YD, Zhang C, Zhang GP, et al. Analysis of endoscopical profiles in the patients with Henoch-Schonlein purpura[J]. China Journal of Leprosy and Skin Diseases, 2017, 33(1):26-28.
[32]Li M, Omi T, Matano Y, et al. The diagnostic usefulness of video capsule endoscopy in adolescent immunoglobulin A vasculitis (Henoch-Schonlein purpura)[J]. J Nippon Med Sch, 2014, 81(2):114-117.
[33]Jennette JC, Falk RJ. Small-vessel vasculitis[J]. N Engl J Med, 1997, 337(21):1512-1523. doi: 10.1056/NEJM199711203372106.
[34]Sneller MC, Fauci AS. Pathogenesis of vasculitis syndromes[J]. Med Clin North Am, 1997, 81(1):221-242. doi: 10.1016/s0025-7125(05)70512-5.
[35]Kato S, Ebina K, Naganuma H, et al. Intestinal IgA deposition in Henoch-Schönlein purpura with severe gastro-intestinal manifestations[J]. Eur J Pediatr, 1996, 155(2):91-95. doi: 10.1007/bf02075757.
[36]Lamireau T, Rebouissoux L, Hehunstre JP. Intravenous immunoglobulin therapy for severe digestive manifestations of Henoch-Schönlein purpura[J]. Acta Pediatr, 2001, 90(9):1081-1082. doi: 10.1111/j.1651-2227.2001.tb01370.x.
[37]Kusuda A, Migita K, Tsuboi M, et al. Successful treatment of adult-onset Henoch-Schönlein purpura nephritis with high-dose immunoglobulins[J]. Intern Med, 1999, 38(4):376-379. doi: 10.2169/internalmedicine.38.376.
