原发性肝癌是目前处于我国常见恶性肿瘤第4位,且是第2位肿瘤相关致死病因,严重威胁着我国人民的生命和健康[1-2]。肝细胞癌(hepatocellular carcinoma,HCC)是全球癌症相关死亡的主要原因之一,中国约占50%的病例和死亡人数[2]。目前,肝切除是HCC的主要治疗手段之一[3]。不幸的是,肝切除术后HCC复发仍然是治疗失败和癌症相关死亡的主要原因。HCC的复发可能源于原发肿瘤的肝内转移,也可能源于残肝的新生肿瘤,分别导致早期和晚期(以24个月为截止时间)的复发[4]。肝切除术后早期复发的HCC(约占HCC复发的70%)较晚期复发更具侵袭性,预后差,是威胁HCC患者生命的主要原因[5]。HCC是一种炎症驱动型癌症,炎症已被证实与分化不良、微血管侵犯(microvascular invasion,MVI)和微转移相关[6-7]。此外,据报道[5,8-9],侵袭性病理特征与HCC有关。因此,炎症可能在预测HCC中发挥重要作用。
炎症被认为是癌症的一个标志[10],越来越多的证据表明,宿主炎症反应与癌症进展和患者生存有关[11]。伴有重型肝炎和活动性炎症的HCC患者,肝切除术后肝内复发率和肝外转移率较高[12-13]。此外,除了肿瘤特征和肝功能等临床参数外,炎症相关标志物是肝切除术后预后的重要预测因素[13]。最近,有许多基于炎症的预后评分是建立在全身炎症反应上的,例如中性粒细胞/淋巴细胞比率(neutrophil to lymphocyte ratio,NLR),血小板与淋巴细胞比(platelet to lymphocyte ratio,PLR),淋巴细胞/单核细胞比(lymphocyte to monocyte ratio,LMR),这些指标显示了对几种癌症的预后价值。包括肺癌、肾细胞癌、生殖细胞瘤、膀胱癌,胃肠道肿瘤和HCC[14-23]。尽管在各种恶性肿瘤中存在淋巴细胞浸润与预期寿命的显著增加有关,但来自循环单核细胞的肿瘤相关巨噬细胞(tumor-associated macrophages,TAM)与预后不良有关[24-27]。这提示LMR可能与癌症的预后有关。值得注意的是,LMR在切除的原发性结直肠腺癌中的预后价值最近已在一项大型回顾性研究中得到证实[28]。
本研究旨在其一探讨术前LMR作为HCC患者行肝切除术的预后价值,以及其对无病生存率(disease-free survival,DFS)和总生存率(overall survival,OS)的影响。
1 资料与方法
1.1 临床资料
回顾性收集了2012年1月—2016年12月在南京中医药大学附属南京医院(南京市第二医院)行根治性肝切除术的88例HCC患者的资料。纳入标准如下:⑴ 经病理证实的HCC诊断;⑵ 术前未行放射与化学治疗;⑶ 美国东部肿瘤协作组评分≤2;⑷ 肿瘤切除边缘阴性;⑸ 无其他恶性肿瘤病史。排除标准如下:⑴ 肝外转移,原发性肝癌伴有肝静脉或下腔静脉或门静脉或动脉或胆道系统侵犯;⑵ 肝切除术后失访。对每位患者的电子病例记录进行查询,例如标准的基线统计资料、原发结肿瘤特征、复发日期、死亡时间和最后一次随访等。
1.2 检验方法
炎症指标均在术前1个月内获得,同时白细胞计数在正常范围内(脾功能亢进除外)。考虑到急性感染或炎症过程改变的可能性而非慢性癌症相关的结果,白细胞减少和白细胞增多患者的炎症指标被排除在分析之外,但是脾功能亢进引起的白细胞减少在排除上述可能后纳入标本中。在HCC的诊断检查中,使用每位患者2 mL静脉血进行全血计数。在乙二胺四乙酸抗凝真空管中采集血液,提取后立即与抗凝剂混合,以防止血栓的形成。采用血液分析仪(XN-9000,Sysmex®,Kobe,Japan)进行电子细胞计数法。本研究通过中性粒细胞对淋巴细胞的绝对计数计算NLR,血小板对淋巴细胞的绝对计数计算PLR,淋巴细胞对单核细胞的绝对计数计算LMR。本研究符合赫尔辛基宣言的标准,经南京中医药大学附属南京医院伦理委员会研究批准,并获得所有患者的知情同意。
1.3 随访
术后随访时间从术后1个月开始执行,术后2年内每3~4个月1次。术后2年以上每6个月1次,直至死亡或失访。随访包括体格检查、血清甲胎蛋白(AFP)和至少1次腹部图像扫描,包括腹部超声、腹部CT或MRI。同时每年进行1次胸部CT平扫。如经腹部超声检查怀疑复发,则行腹部增强CT或增强MRI或腹部超声造影检查以确诊,胸部增强CT扫描排除肺转移。骨扫描或正电子发射CT检查排除其他部位可以转移病灶。肿瘤复发的诊断标准如下:⑴ 肝活检;⑵ CT/MRI的典型表现(动脉期强化,门脉期/静脉期减弱)[29]。
1.4 统计学处理
统计分析采用SPSS 17.0(SPSS,Chicago,IL,USA)和Prism 5(GraphPad Software La Jolla,CA,USA)。DFS的计算从肝切除之日起至疾病复发之日止,并在与癌症死亡无关时或在患者最后一次随访时(如果患者在此期间仍无肿瘤)进行截尾。OS的计算从肝切除术之日起至因任何原因死亡之日止,并在最后一次随访时对仍在世的患者进行删失。通过应用受试者工作曲线分析(ROC),确定LMR、PLR、NLR的最佳截断水平。χ2检验用于计算临床病理分类特征与LMR之间的关系。Kaplan-Meier方法分析LMR对DFS和OS的影响。各组生存结果采用log-rank检验进行比较。采用前向逐步法Cox回归模型,将单变量分析中的重要变量纳入多变量分析。P<0.05为差异有统计学意义。
2 结 果
2.1 LMR、PLR和NLR的ROC曲线分析结果
根据LMR、PLR和NLR与OS的关系绘制ROC曲线以确定最佳诊断界值,本研究中,当LMR为2.87时,曲线下面积(AUC)为0.757,敏感度为76.5%,特异度为75.7%。当PLR为133.61时,AUC为0.583,敏感度为29.7%,特异度为86.3%。当NLR为2.60时,AUC为0.687敏感度为64.9%,特异度为72.5%(图1)(表1),同时通过Spearson相关分析显示,淋巴细胞计数与单核细胞计数存在一定的相关性(rs=0.652,P<0.001)(图2)。
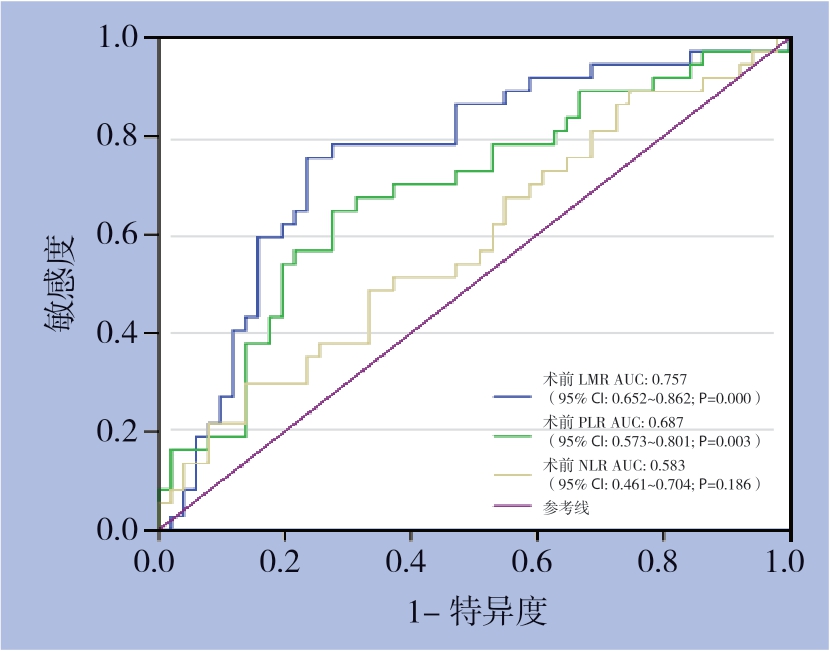
图1 LMR、PLR、NLR的预测HCC患者生存的ROC曲线
Figure 1 ROC curves of LMR,PLR and NLR for predicting the survival of HCC patients

图2 淋巴细胞绝对值与单核细胞绝对值相关分析结果
Figure 2 Correlation analysis between the absolute values of lymphocytes and monocytes
表1 LMR、PLR、NLR的ROC曲线分析结果
Table 1 Results of analysis of ROC curves of LMR,PLR and NLR
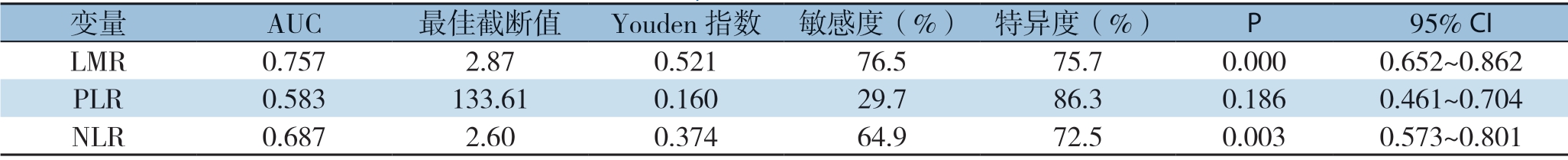
变量 AUC 最佳截断值 Youden指数 敏感度(%) 特异度(%) P 95% CI LMR 0.757 2.87 0.521 76.5 75.7 0.000 0.652~0.862 PLR 0.583 133.61 0.160 29.7 86.3 0.186 0.461~0.704 NLR 0.687 2.60 0.374 64.9 72.5 0.003 0.573~0.801
2.2 LMR与患者临床病理特征资料的相关分析
根据上述ROC分析结果,将患者分成两组,LMR>2.87为高LMR组和LMR≤2.87为低LMR组。结果显示,LMR分组与巴塞罗那临床肝癌分期(barcelona clinic liver cancer,BCLC)和中国肝癌分期(china liver cancer staging,CNLC)及其他临床病理因素均无明显关系(均P>0.05),仅发现LMR与患者肿瘤数目有关(P=0.048) (表2)。
表2 LMR与HCC患者临床病理特征关系[n(%)]
Table 2 Relations of LMR with the clinicopathologic features of HCC patients [n (%)]
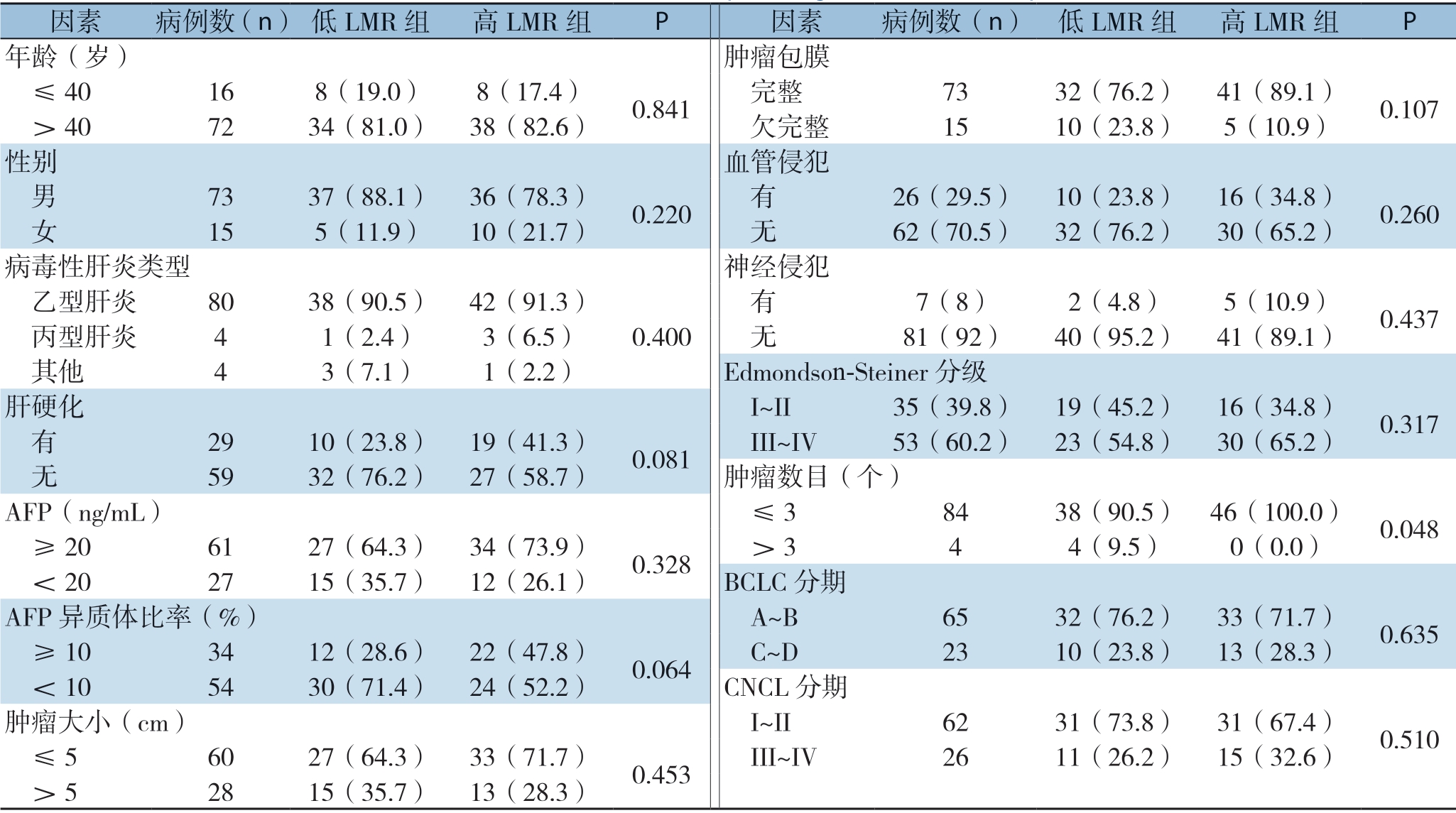
因素 病例数(n)低LMR组 高LMR组 P 因素 病例数(n) 低LMR组 高LMR组 P年龄(岁) 肿瘤包膜 ≤40 16 8(19.0) 8(17.4) 0.841 完整 73 32(76.2) 41(89.1) 0.107 >40 72 34(81.0) 38(82.6) 欠完整 15 10(23.8) 5(10.9)性别 血管侵犯 男 73 37(88.1) 36(78.3) 0.220 有 26(29.5) 10(23.8) 16(34.8) 0.260 女 15 5(11.9) 10(21.7) 无 62(70.5) 32(76.2) 30(65.2)病毒性肝炎类型 神经侵犯 乙型肝炎 80 38(90.5) 42(91.3)0.400 有 7(8) 2(4.8) 5(10.9) 0.437 丙型肝炎 4 1(2.4) 3(6.5) 无 81(92) 40(95.2) 41(89.1) 其他 4 3(7.1) 1(2.2) Edmondson-Steiner分级肝硬化 I~II 35(39.8) 19(45.2) 16(34.8) 0.317 有 29 10(23.8) 19(41.3) 0.081 III~IV 53(60.2) 23(54.8) 30(65.2) 无 59 32(76.2) 27(58.7) 肿瘤数目(个)AFP(ng/mL) ≤3 84 38(90.5) 46(100.0) 0.048 ≥20 61 27(64.3) 34(73.9) 0.328 >3 4 4(9.5) 0(0.0)<20 27 15(35.7) 12(26.1) BCLC分期AFP异质体比率(%) A~B 65 32(76.2) 33(71.7) 0.635 ≥10 34 12(28.6) 22(47.8) 0.064 C~D 23 10(23.8) 13(28.3)<10 54 30(71.4) 24(52.2) CNCL分期肿瘤大小(cm) I~II 62 31(73.8) 31(67.4) 0.510 ≤5 60 27(64.3) 33(71.7) 0.453 III~IV 26 11(26.2) 15(32.6) >5 28 15(35.7) 13(28.3)
2.3 LMR与患者预后的相关生存分析
为了进一步分析LMR与HCC患者预后的关系,根据LMR截断值将88例患者分成高LMR组和低LMR组。然后,绘制Kaplan-Meier生存曲线,使用log-rank检验比较生存率,进一步研究OS和DFS的关系。结果显示,高LMR组患者的DFS(P=0.001)与OS(P=0.000)均明显优于低LMR组 (图3)。结合肿瘤BCLC分期与CNLC分期,将早期肿瘤包括CNLC I/II期和BCLC A/B期,晚期肿瘤包括CNLC III/IV期和BCLC期C/D[31]分别比较。结果显示,无论是BCLC A/B期患者还是BCLC C/D期患者中,高LMR组患者的DFS与OS均明显优于低LMR组(均P<0.05)。在CNLC I/II期患者中,高LMR组与低LMR组的DFS无明显差异(P=0.132),但高LMR组的OS优于低LMR组(P=0.005);在CNLC III/IV期患者中,高LMR组患者的DFS(P=0.001)与OS(P=0.000)均明显优于低LMR组(均P<0.05)(图4-5)。
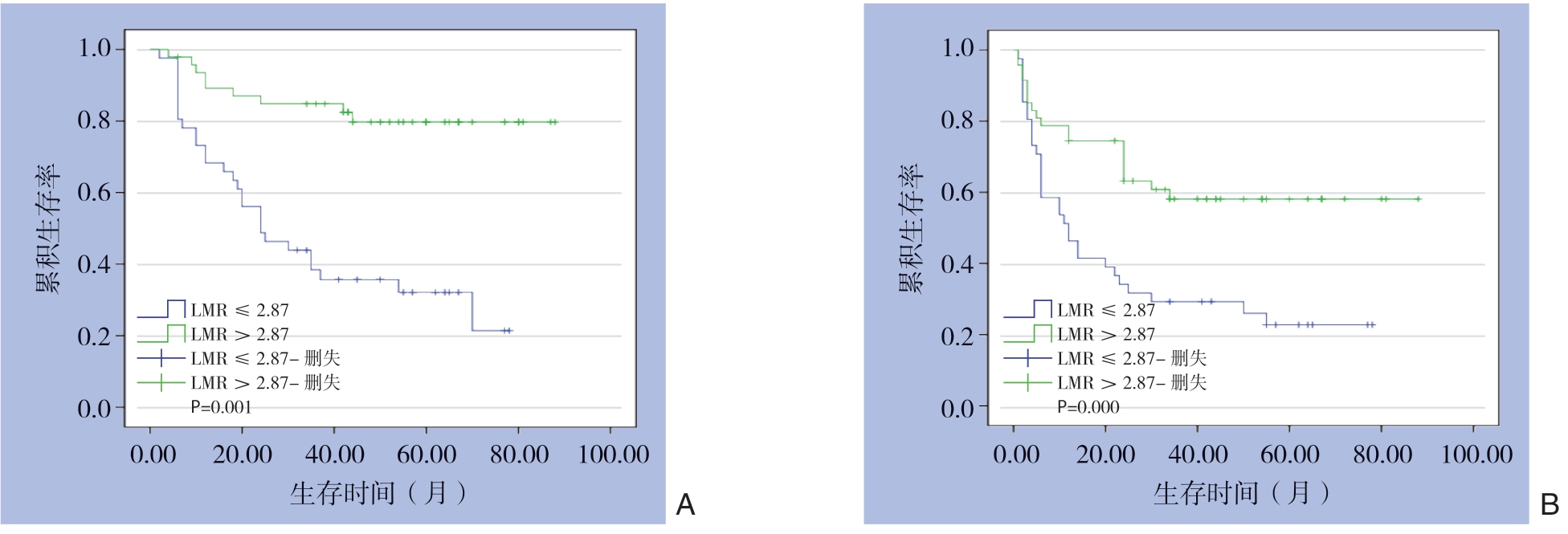
图3 高LMR组与低LMR组HCC患者的生存曲线
Figure 3 Survival curves of HCC patients in high LMR group and low LMR group
A:DFS曲线;B:OS曲线
A:DFS curves;B:OS curves

图4 BCLC分期分层后高LMR组与低LMR组HCC患者的生存曲线
Figure 4 Survival curves of HCC patients in high LMR group and low LMR group after BCLC stage stratification
A:BCLC A/B期患者DFS曲线;B:BCLC A/B期患者OS曲线;C:BCLC C/D期患者DFS曲线;D:BCLC C/D期患者OS曲线
A:DFS curves of patients classified as BCLC A/B stage;B:OS curves of patients classified as BCLC A/B stage;C:DFS curves of patients classified as BCLC C/D stage;D:OS curves of patients classified as BCLC C/D stage
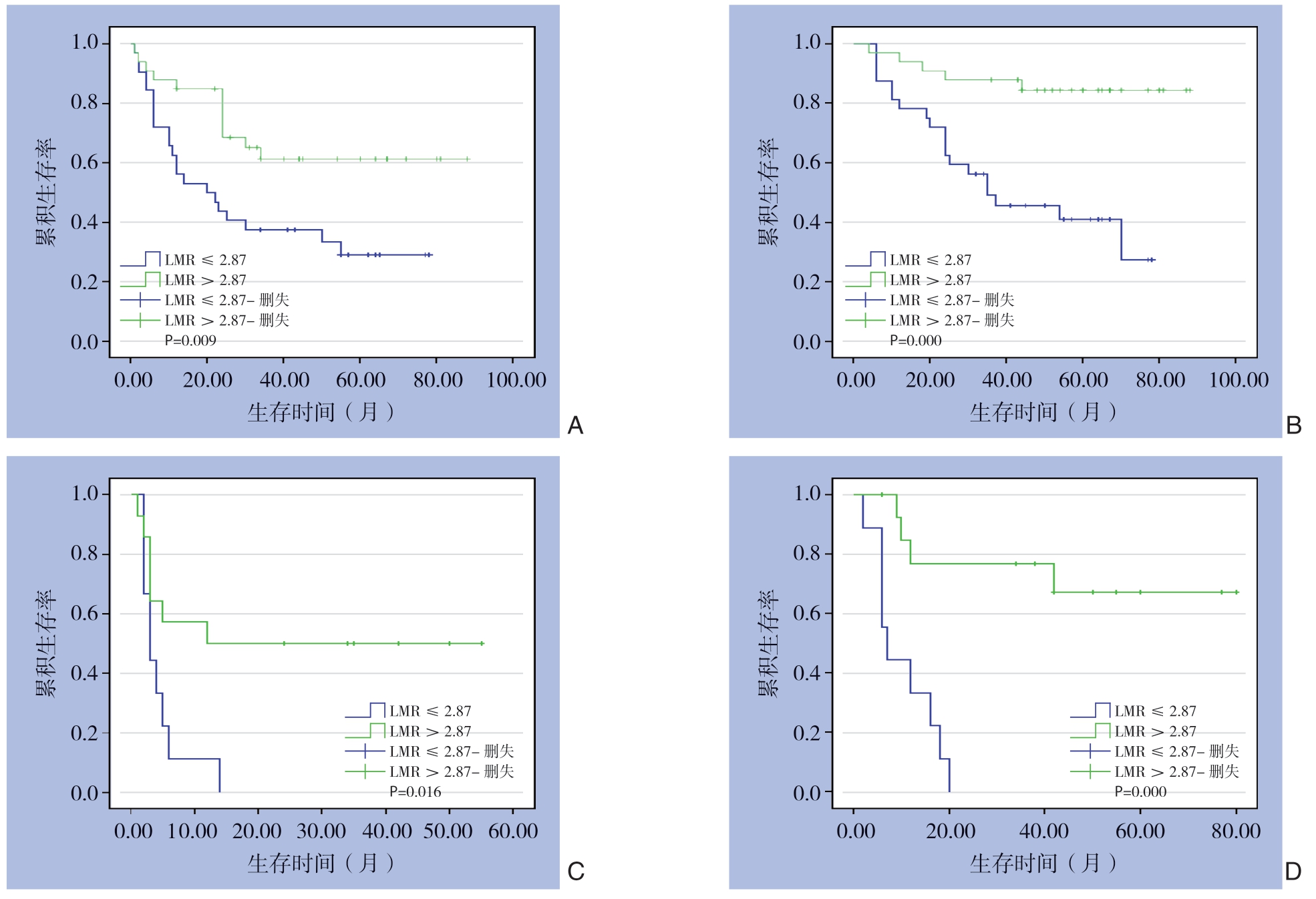
图5 CNLC分期分层后高LMR组与低LMR组HCC患者的生存曲线
Figure 5 Survival curves of HCC patients in high LMR group and low LMR group after CNLC stage stratification
A:CNLC I/II期患者DFS曲线;B:CNLC I/II期患者OS曲线;C:CNLC III/IV期者DFS曲线;D:CNLC III/IV期者OS曲线
A:DFS curves of patients classified as CNLC I/II stage;B:OS curves of patients classified asCNLC I/II stage;C:DFS curves of patients classified as CNLC III/IV stage;D:OS curves of patients classified as CNLC III/IV stage
2.4 HCC患者生存的影响因素分析
2.4.1 DFS的影响因素分析 单因素分析提示肿瘤大小(P=0.028)、血管侵犯(P=0.039)、CNLC分期(P=0.018)、BCLC分期(P=0.011)及LMR(P=0.002)有预后价值;通过采用前向逐步法Cox回归模型,将单因素分析中的重要变量纳入多因素分析提示LMR(P=0.001)为HCC患者DFS的独立影响因素(表3)。
2.4.2 OS的影响因素分析 单因素分析提示肿瘤大小(P=0.011)、CNLC分期(P=0.043)、BCLC分期(P=0.021)及LMR(P=0.000)有预后价值。通过采用前向逐步法Cox回归模型,将单因素分析中的重要变量纳入多因素分析提示只有BCLC分期(P=0.000)和LMR(P=0.000)为HCC患者OS的独立影响因素(表4)。为进一步了解LMR在HCC患者中的预后价值,将LMR、PLR以及NLR三者作为连续变量(试图校正因为截断值对变量的影响造成结果的偏差)进行Cox比例风险分析[32],仅LMR具有预后价值(HR=0.571,95% CI=0.416~0.786,P=0.001)(表5)。
表3 HCC患者DFS的单因素和多因素Cox比例风险回归分析
Table 3 Univariate and multivariate Cox proportional hazards regression analysis of factors for DFS of HCC patients
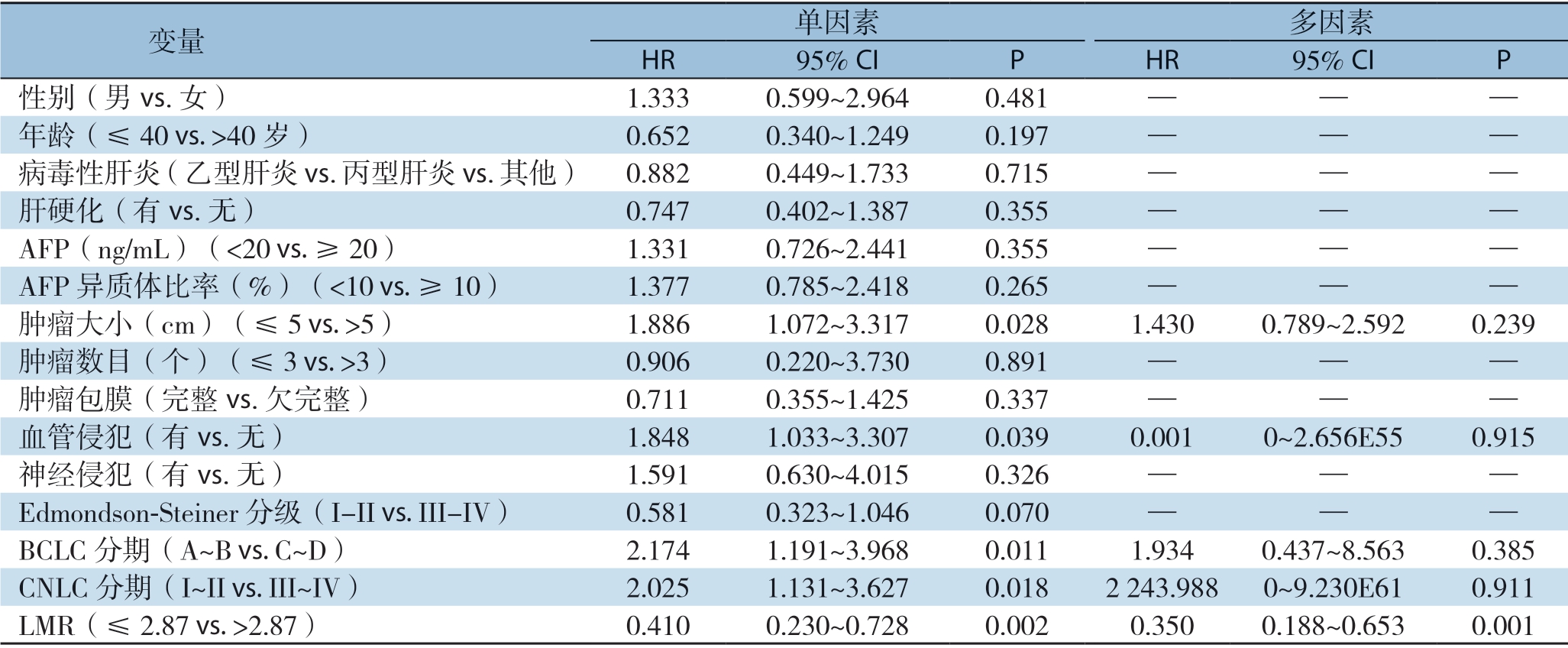
变量 单因素 多因素HR 95% CI P HR 95% CI P性别(男vs.女) 1.333 0.599~2.964 0.481 — — —年龄(≤40 vs.>40岁) 0.652 0.340~1.249 0.197 — — —病毒性肝炎(乙型肝炎vs.丙型肝炎vs.其他) 0.882 0.449~1.733 0.715 — — —肝硬化(有vs.无) 0.747 0.402~1.387 0.355 — — —AFP(ng/mL)(<20 vs.≥20) 1.331 0.726~2.441 0.355 — — —AFP异质体比率(%)(<10 vs.≥10) 1.377 0.785~2.418 0.265 — — —肿瘤大小(cm)(≤5 vs.>5) 1.886 1.072~3.317 0.028 1.430 0.789~2.592 0.239肿瘤数目(个)(≤3 vs.>3) 0.906 0.220~3.730 0.891 — — —肿瘤包膜(完整vs.欠完整) 0.711 0.355~1.425 0.337 — — —血管侵犯(有vs.无) 1.848 1.033~3.307 0.039 0.001 0~2.656E55 0.915神经侵犯(有vs.无) 1.591 0.630~4.015 0.326 — — —Edmondson-Steiner分级(I-II vs.III-IV) 0.581 0.323~1.046 0.070 — — —BCLC分期(A~B vs.C~D) 2.174 1.191~3.968 0.011 1.934 0.437~8.563 0.385 CNLC分期(I~II vs.III~IV) 2.025 1.131~3.627 0.018 2 243.988 0~9.230E61 0.911 LMR(≤2.87 vs.>2.87) 0.410 0.230~0.728 0.002 0.350 0.188~0.653 0.001
表4 HCC患者OS的单因素和多因素Cox比例风险回归分析
Table 4 Univariate and multivariate Cox proportional hazards regression analysis of factors for OS of HCC patients
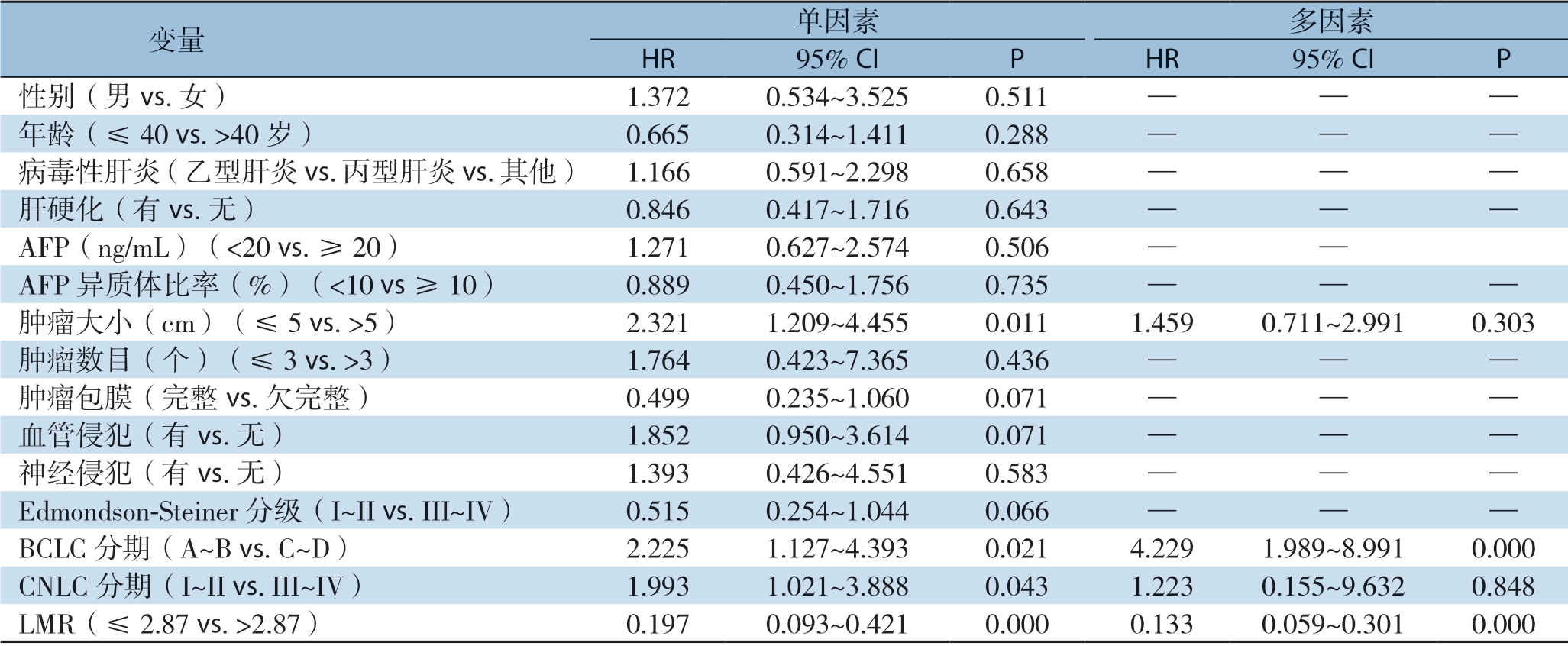
变量 单因素 多因素HR 95% CI P HR 95% CI P性别(男vs.女) 1.372 0.534~3.525 0.511 — — —年龄(≤40 vs.>40岁) 0.665 0.314~1.411 0.288 — — —病毒性肝炎(乙型肝炎vs.丙型肝炎vs.其他) 1.166 0.591~2.298 0.658 — — —肝硬化(有vs.无) 0.846 0.417~1.716 0.643 — — —AFP(ng/mL)(<20 vs.≥20) 1.271 0.627~2.574 0.506 — —AFP异质体比率(%)(<10 vs ≥10) 0.889 0.450~1.756 0.735 — — —肿瘤大小(cm)(≤5 vs.>5) 2.321 1.209~4.455 0.011 1.459 0.711~2.991 0.303肿瘤数目(个)(≤3 vs.>3) 1.764 0.423~7.365 0.436 — — —肿瘤包膜(完整vs.欠完整) 0.499 0.235~1.060 0.071 — — —血管侵犯(有vs.无) 1.852 0.950~3.614 0.071 — — —神经侵犯(有vs.无) 1.393 0.426~4.551 0.583 — — —Edmondson-Steiner分级(I~II vs.III~IV) 0.515 0.254~1.044 0.066 — — —BCLC分期(A~B vs.C~D) 2.225 1.127~4.393 0.021 4.229 1.989~8.991 0.000 CNLC分期(I~II vs.III~IV) 1.993 1.021~3.888 0.043 1.223 0.155~9.632 0.848 LMR(≤2.87 vs.>2.87) 0.197 0.093~0.421 0.000 0.133 0.059~0.301 0.000
表5 HCC患者OS的LMR、PLR、NLR单因素和多因素Cox比例风险回归分析比较
Table 5 Univariate and multivariate Cox proportional hazards regression analysis of LMR,PLR and NLR for OS of HCC patients
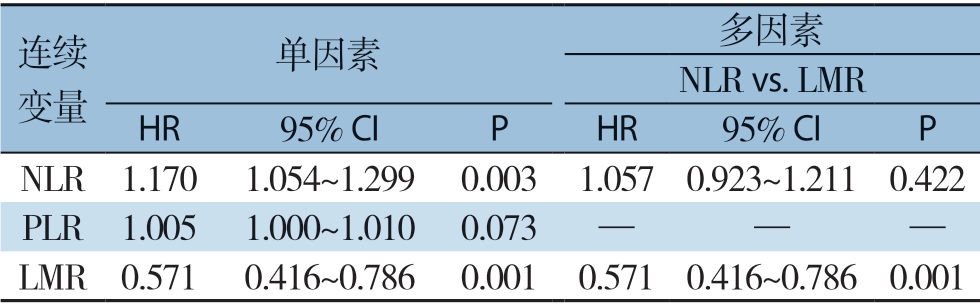
连续变量单因素 多因素NLR vs.LMR HR 95% CI P HR 95% CI P NLR 1.170 1.054~1.299 0.003 1.057 0.923~1.211 0.422 PLR 1.005 1.000~1.010 0.073 — — —LMR 0.571 0.416~0.786 0.001 0.571 0.416~0.786 0.001
3 讨 论
本研究结果表明,术前LMR是HCC根治性切除患者OS和DFS不良预后的独立指标。此外,在CNLC分期或BCLC分期患者的亚组中发现,LMR也与OS和DFS显著相关。且近期有一项研究发现LMR、PLR、NLR与TNM分期及BCLC分期密切相关,这从另一面证实了本研究的结果的价值[33]。同时本研究将LMR与CNLC分期和BCLC分期相结合有助于进一步量化预后风险,并提供更多的预后信息。虽然LMR的预后价值已经在血液病恶性肿瘤和最近的实体肿瘤中得到证实[28,34-36],但LMR在HCC分期亚组中的预后价值报道较少。然而,它的价值需要独立的和更多的数据来验证。
之前发表的关于实体瘤中LMR的数据,一项研究调查了LMR对已切除的原发性结肠直肠癌患者的影响,另一项研究调查了诊断为软组织肉瘤的患者[28,34]。在前一项研究中,Stotz等[28]在372例结直肠癌患者中使用LMR截断值(DFS为2.83,OS为2.14)证明了LMR与DFS和OS之间的联系。在第二项研究中,Szkandera等[34]通过分析340例软组织肉瘤患者,确定了低于2.85的LMR与较差的DFS和OS之间存在相关性。这些截断值与本文当前研究中确定的截断值很接近。且本文为了进一步了解LMR在HCC中的预后价值,根据CNLC分期及BCLC分期特点进一步分析,得到在肿瘤早期(CNLC I~II,BCLC A~B)及肿瘤晚期(CNLC III~IV,BCLC C~D)DFS和OS的差异。且通过DFS及OS的Cox多因素比例风险回归分析得到LMR可作为HCC独立预后危险因素。
通过ROC曲线分析中Youden指数为LMR、NLR和PLR定义了最佳切割点,分别为2.87、2.60和133.61。同时发现LMR(AUC:0.757)预测患者生存的效能大于NLR(AUC=0.687)和PLR(AUC=0.583)。此外,本研究还发现,与众所周知的OS和DFS的独立指标NLR和PLR相比,LMR是一个更好的长期生存指标[37-40]。在此研究之前,相关研究通常报道NLR优于PLR[41-44]。然而,本研究发现NLR和PLR都不是OS的独立指标。在本研究中,在单因素和多因素分析中发现,LMR高于2.87与HCC患者的OS和DFS增加之间存在明显联系。
LMR降低与患者不良预后相关的机制尚不清楚,但一些研究已经给出了一些解释。早在20世纪70年代,在对晚期肿瘤患者淋巴细胞减少的研究中,这些淋巴细胞成分的标记物已经得到了充分的证实[45]。这是其他已建立的炎症标志物的基础之一,如PLR和NLR。淋巴细胞是免疫系统的支柱,是在乙肝或者丙肝病毒、酒精等毒性物质或代谢综合征等慢性炎症背景下发生肝细胞癌的微环境的关键防御机制[46-47]。肿瘤浸润淋巴细胞的预后意义已被公认[48]。虽然单核细胞与预后的相关性最近已被评估,但只有初步的假设可以解释为什么单核细胞可能提示预后信息。在此,笔者提出了几种可能的机制。首先,已有研究发现循环单核细胞可能促进肿瘤生长,帮助肿瘤细胞逃避免疫监视[49-50]。其次,据报道[51-53],来源于循环单核细胞的TAM能够渗透到HCC基质中,发挥包括促进增殖、转移、血管生成和免疫抑制在内的活性。
本文虽然提供了一些新的发现,但是这项研究有几个局限性需要解决。一方面,由于LMR的值在随访时是动态的,本研究仅使用基线值来预测未来的结果,因此可能会遗漏很多信息,且样本量较小,但近期Yu等[33]发表文献报告6 619例数据,研究结果与本文类似,可为本研究提供一定的研究基础。另一方面,由于回顾性研究的性质,存在潜在的选择偏差。
综上所述,本研究表明LMR优于NLR和PLR作为OS和DFS的独立预后因素,具有一定的临床价值,外科医生可以利用LMR结合HCC分期对患者进行危险分级,判断患者是否为高危人群。本研究结果尚需要一个更大的前瞻性研究来进一步证实。
[1]Bray F,Ferlay J,Soerjomataram I,et al.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J].CA Cancer J Clin,2018,68(6):394-424.doi:10.3322/caac.21492.
[2]Zhou M,Wang H,Zeng X,Mortality,morbidity,and risk factors in China and its provinces,1990-2017:a systematic analysis for the Global Burden of Disease Study 2017[J].Lancet,2019,394(10204):1145-1158.doi:10.1016/S0140-6736(19)30427-1.
[3]Forner A,Reig M,Bruix J.Hepatocellular carcinoma[J].Lancet,2018,391(10127):1301-1314.doi:10.1016/S0140-6736(18)30010-2.
[4]Portolani N,Coniglio A,Ghidoni S,et al.Early and late recurrence after liver resection for hepatocellular carcinoma:prognostic and therapeutic implications[J].Ann Surg,2006,243(2):229-235.doi:10.1097/01.sla.0000197706.21803.a1.
[5]Tabrizian P,Jibara G,Shrager B,et al.Recurrence of hepatocellular cancer after resection:patterns,treatments,and prognosis[J].Ann Surg,2015,261(5):947-955.doi:10.1097/SLA.0000000000000710.
[6]Cabillic F,Corlu A.Regulation of Transdifferentiation and Retrodifferentiation by Inflammatory Cytokines in Hepatocellular Carcinoma[J].Gastroenterology,2016,151(4):607-615.doi:10.1053/j.gastro.2016.06.052.
[7]Zheng J,Seier K,Gonen M,et al.Utility of Serum Inflammatory Markers for Predicting Microvascular Invasion and Survival for Patients with Hepatocellular Carcinoma[J].Ann Surg Oncol,2017,24(12):3706-3714.doi:10.1245/s10434-017-6060-7.
[8]Imamura H,Matsuyama Y,Tanaka E,et al.Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy[J].J Hepatol,2003,38(2):200-207.doi:10.1016/s0168-8278(02)00360-4.
[9]Chan A,Zhong J,Berhane S,et al.Development of pre and postoperative models to predict early recurrence of hepatocellular carcinoma after surgical resection[J].J Hepatol,2018,69(6):1284-1293.doi:10.1016/j.jhep.2018.08.027.
[10]Hanahan D,Weinberg RA.Hallmarks of cancer:the next generation[J].Cell,2011,144(5):646-674.doi:10.1016/j.cell.2011.02.013.
[11]Diakos CI,Charles KA,McMillan DC,et al.Cancer-related inflammation and treatment effectiveness[J].Lancet Oncol,2014,15(11):e493-503.doi:10.1016/S1470-2045(14)70263-3.
[12]Wu TJ,Chang SS,Li CW,et al.Severe Hepatitis Promotes Hepatocellular Carcinoma Recurrence via NF-kappaB Pathway-Mediated Epithelial-Mesenchymal Transition after Resection[J].Clin Cancer Res,2016,22(7):1800-1812.doi:10.1158/1078-0432.CCR-15-0780.
[13]Chen L,Zhang Q,Chang W,et al.Viral and host inflammationrelated factors that can predict the prognosis of hepatocellular carcinoma[J].Eur J Cancer,2012,48(13):1977-1987.doi:10.1016/j.ejca.2012.01.015.
[14]Kinoshita A,Onoda H,Imai N,et al.Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma[J].Br J Cancer,2012,107(6):988-993.doi:10.1038/bjc.2012.354.
[15]Halazun KJ,Hardy MA,Rana AA,et al.Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma[J].Ann Surg,2009,250(1):141-151.doi:10.1097/SLA.0b013e3181a77e59.
[16]Szkandera J,Absenger G,Liegl-Atzwanger B,et al.Elevated preoperative neutrophil/lymphocyte ratio is associated with poor prognosis in soft-tissue sarcoma patients[J].Br J Cancer,2013,108(8):1677-1683.doi:10.1038/bjc.2013.135.
[17]Chang Y,An H,Xu L,et al.Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma[J].Br J Cancer,2015,113(4):626-633.doi:10.1038/bjc.2015.241.
[18]Chovanec M,Cierna Z,Miskovska V,et al.Systemic immuneinflammation index in germ-cell tumours[J].Br J Cancer,2018,118(6):831-838.doi:10.1038/bjc.2017.460.
[19]Hu B,Yang X R,Xu Y,et al.Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma[J].Clin Cancer Res,2014,20(23):6212-6222.doi:10.1158/1078-0432.CCR-14-0442.
[20]Forrest LM,McMillan D C,McArdle CS,et al.Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer[J].Br J Cancer,2004,90(9):1704-1706.doi:10.1038/sj.bjc.6601789.
[21]Proctor MJ,Morrison DS,Talwar D,et al.A comparison of inflammation-based prognostic scores in patients with cancer.A Glasgow Inflammation Outcome Study[J].Eur J Cancer,2011,47(17):2633-2641.doi:10.1016/j.ejca.2011.03.028.
[22]Hu G,Liu Q,Ma JY,et al.Prognostic Significance of Platelet-to-Lymphocyte Ratio in Cholangiocarcinoma:A Meta-Analysis[J].Biomed Res Int,2018,2018:7375169.doi:10.1155/2018/7375169.
[23]Ma JY,Hu G,Liu Q.Prognostic Significance of the Lymphocyteto-Monocyte Ratio in Bladder Cancer Undergoing Radical Cystectomy:A Meta-Analysis of 5638 Individuals[J].Dis Markers,2019,2019:7593560.doi:10.1155/2019/7593560.
[24]Kawai O,Ishii G,Kubota K,et al.Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer[J].Cancer,2008,113(6):1387-1395.doi:10.1002/cncr.23712.
[25]Corthay A.Does the immune system naturally protect against cancer?[J].Front Immunol,2014,5:197.doi:10.3389/fimmu.2014.00197.
[26]Balermpas P,Rödel F,Liberz R,et al.Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences[J].Br J Cancer,2014,111(8):1509-1518.doi:10.1038/bjc.2014.446.
[27]Bingle L,Brown NJ,Lewis CE.The role of tumour-associated macrophages in tumour progression:implications for new anticancer therapies[J].J Pathol,2002,196(3):254-265.doi:10.1002/path.1027.
[28]Stotz M,Pichler M,Absenger G,et al.The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer[J].Br J Cancer,2014,110(2):435-440.doi:10.1038/bjc.2013.785.
[29]European Association for the Study of the Liver,Electronic address:easloffice@easloffice.eu,European Association for the Study of the Liver.EASL Clinical Practice Guidelines:Management of hepatocellular carcinoma[J].J Hepatol,2018,69(1):182-236.doi:10.1016/j.jhep.2018.03.019.
[30]中华人民共和国国家卫生健康委员会医政医管局.原发性肝癌诊疗规范(2019年版)[J].中华消化外科杂志,2020,19(1):1-20.doi:10.3760/cma.j.issn.1673-9752.2020.01.001.
Bureau of Medical Administration,National Health and Family Planning Committee.Standardization for diagnosis and treatment of hepatocellular carcinoma (2019 edition) [J].Chinese Journal of Digestive Surgery,2020,19(1):1-20.doi:10.3760/cma.j.issn.1673-9752.2020.01.001.
[31]Shu QH,Ge YS,Ma HX,et al.Prognostic value of polarized macrophages in patients with hepatocellular carcinoma after curative resection[J].J Cell Mol Med,2016,20(6):1024-1035.doi:10.1111/jcmm.12787.
[32]Neofytou K,Smyth EC,Giakoustidis A,et al.The Preoperative Lymphocyte-to-Monocyte Ratio is Prognostic of Clinical Outcomes for Patients with Liver-Only Colorectal Metastases in the Neoadjuvant Setting[J].Ann Surg Oncol,2015,22(13):4353-4362.doi:10.1245/s10434-015-4481-8.
[33]Yu JI,Park HC,Yoo GS,et al.Clinical Significance of Systemic Inflammation Markers in Newly Diagnosed,Previously Untreated Hepatocellular Carcinoma[J].Cancers (Basel),2020,12(5):1300.doi:10.3390/cancers12051300.
[34]Szkandera J,Gerger A,Liegl-Atzwanger B,et al.The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas[J].Int J Cancer,2014,135(2):362-370.doi:10.1002/ijc.28677.
[35]Schmidt H,Bastholt L,Geertsen P,et al.Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma:a prognostic model[J].Br J Cancer,2005,93(3):273-278.doi:10.1038/sj.bjc.6602702.
[36]Porrata LF,Ristow K,Colgan JP,et al.Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin's lymphoma[J].Haematologica,2012,97(2):262-269.doi:10.3324/haematol.2011.050138.
[37]Yang HJ,Guo Z,Yang YT,et al.Blood neutrophil-lymphocyte ratio predicts survival after hepatectomy for hepatocellular carcinoma:A propensity score-based analysis[J].World J Gastroenterol,2016,22(21):5088-5095.doi:10.3748/wjg.v22.i21.5088.
[38]Li X,Chen ZH,Xing YF,et al.Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma[J].Tumour Biol,2015,36(4):2263-2269.doi:10.1007/s13277-014-2833-9.
[39]Mano Y,Shirabe K,Yamashita Y,et al.Preoperative neutrophil-tolymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma:a retrospective analysis[J].Ann Surg,2013,258(2):301-305.doi:10.1097/SLA.0b013e318297ad6b.
[40]Okamura Y,Sugiura T,Ito T,et al.Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma[J].Br J Surg,2016,103(7):891-898.doi:10.1002/bjs.10123.
[41]Yang T,Zhu J,Zhao L,et al.Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection[J].J Surg Oncol,2017,115(6):718-728.doi:10.1002/jso.24549.
[42]Ji F,Liang Y,Fu SJ,et al.A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection:the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI)[J].BMC Cancer,2016,16:137.doi:10.1186/s12885-016-2189-1.
[43]Yamamura K,Sugimoto H,Kanda M,et al.Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection[J].J Hepatobiliary Pancreat Sci,2014,21(9):682-688.doi:10.1002/jhbp.114.
[44]任家书,马秀现,李健,等.术前中性粒细胞/淋巴细胞比值对小肝癌患者并发微血管浸润的预测价值[J].中国普通外科杂志,2018,27(7):840-846.doi:10.3978/j.issn.1005-6947.2018.07.007.
Ren JS,Ma XX,Li J,et al.Value of preoperative neutrophil to lymphocyte ratio for prediction of microvascular invasion patients with small hepatocellular carcinoma[J].Chinese Journal of General Surgery,2018,27(7):840-846.doi:10.3978/j.issn.1005-6947.2018.07.007.
[45]Kim US,Papatestas AE.Letter:Peripheral lymphocyte counts in colonic disease[J].Lancet,1974,2(7878):462-463.doi:10.1016/s0140-6736(74)91843-1.
[46]Wang Y,Zhang C.The Roles of Liver-Resident Lymphocytes in Liver Diseases[J].Front Immunol,2019,10:1582.doi:10.3389/fimmu.2019.01582.
[47]Kim JH,Jenrow KA,Brown SL.Novel biological strategies to enhance the radiation therapeutic ratio[J].Radiat Oncol J,2018,36(3):172-181.doi:10.3857/roj.2018.00332.
[48]Yao W,He JC,Yang Y,et al.The Prognostic Value of Tumorinfiltrating Lymphocytes in Hepatocellular Carcinoma:a Systematic Review and Meta-analysis[J].Sci Rep,2017,7(1):7525.doi:10.1038/s41598-017-08128-1.
[49]Augier S,Ciucci T,Luci C,et al.Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance[J].J Immunol,2010,185(12):7165-7173.doi:10.4049/jimmunol.0902583.
[50]Hu YC,Yi ZJ,Zhou Y,et al.Overexpression of RIP140 suppresses the malignant potential of hepatocellular carcinoma by inhibiting NFkappaBmediated alternative polarization of macrophages[J].Oncol Rep,2017,37(5):2971-2979.doi:10.3892/or.2017.5551.
[51]Galdiero MR,Bonavita E,Barajon I,et al.Tumor associated macrophages and neutrophils in cancer[J].Immunobiology,2013,218(11):1402-1410.doi:10.1016/j.imbio.2013.06.003.
[52]Jackaman C,Tomay F,Duong L,et al.Aging and cancer:The role of macrophages and neutrophils[J].Ageing Res Rev,2017,36:105-116.doi:10.1016/j.arr.2017.03.008.
[53]Yan C,Yang Q,Gong Z.Tumor-Associated Neutrophils and Macrophages Promote Gender Disparity in Hepatocellular Carcinoma in Zebrafish [J].Cancer Res,2017,77(6):1395-1407.doi:10.1158/0008-5472.CAN-16-2200.
