肝细胞癌是世界最常见的肿瘤之一,也是全球癌症病死率第二的恶性肿瘤。中国是人口大国,也是肝细胞癌大国,全球每年新发肝细胞癌病患中约有一半以上出自中国[1-2]。迄今为止,外科切除仍是肝细胞癌最有效的治疗方法,但术后复发是影响治疗效果的重要因素[3]。根据复发时间,术后复发可分为早期复发(术后2年内)和晚期复发(2年后)[4-5]。术后早期复发严重影响肝细胞癌患者的预后,肝细胞癌术后2年内死亡的主要原因是肿瘤的早期复发[6]。因此,本研究主要探讨影响肝细胞癌患者术后早期复发的危险因素,为预测复发风险、改善预后提供依据。
1 资料与方法
1.1 研究对象
回顾性分析2011—2016年华中科技大学同济医学院附属同济医院就诊的225例肝细胞癌患者。纳入标准:(1)年龄1 8 ~7 5 岁,性别不限; (2) 临床诊断为肝细胞癌, 巴塞罗那分期(Barcelona Clinic Liver Cancer,BCLC)A或B期, 行根治性肝切除;(3)Child-Pugh肝功能分级为 A级,或B级但经护肝治疗后恢复至A级;(4) 不伴随其他的恶性疾病;(5) 无严重的心、肺、脑等器官的器质性疾病;⑹ 临床资料齐全;⑺ 自愿签署知情同意书。排除标准:(1) 孕妇及哺乳期妇女;(2) 有器官移植病史;(3)术前行其它抗肿瘤治疗及免疫治疗;(4) 药物滥用者,或患有可能干扰研究依从性的心理或精神等疾病;(5) 无法获得随访资料;⑹ 研究者认为不适合本研究的其它情况。
1.2 分析指标
早期复发患者例数;患者各项基线指标:性别、年龄、肿瘤最大径、肿瘤个数、肝功能分级、HBV-DNA、甲胎蛋白(AFP)水平、血小板计数(PLT)、碱性磷酸酶(ALP)水平、中性粒细胞-淋巴细胞比值(neutrophil to lymphocyte ratio,NLR)、γ-谷氨酰转肽酶(γ-GT)水平、肝切除方式及术中输血与肝门阻断与否等。
1.3 随访
术后半年内每1个月1次,术后2年内每3个月1次。术后3~5年每6个月1次,术后5年以上每1年1次,直至死亡或失访。随访包括体格检查、肿瘤标志物和肝脏影像学检查(肝脏超声、肝脏CT或MRI)。同时每年进行1次胸部CT平扫。
1.4 统计学处理
通过Epidata 3.0建立数据库,应用SPSS 26(IBM)进行数据分析。分类变量用实际频数表示,定量资料用中位数及四分位数来描述。Logistic 回归模型确定独立危险因素,ox 回归模型建立预测模型。运用受试者工作特征曲线(receiver operating characteristic curve,ROC)及曲线下面积(area under curve,AUC)评价预测效能。以α=0.05(双侧)作为检验水平。
2 结 果
2.1 患者早期复发情况及其他基线资料
225例患者中,130例患者在术后2年内复发(早期复发),占57.8%。男179例,占79.6%;中位年龄52岁。201例患者入院时Child-pugh分级为A级,24例患者入院时Child-pugh分级为B级,但经护肝治疗后恢复至A级。肿瘤中位最大径为 3.4(2.4~4.75)cm,191例患者为单发肿瘤,34例患者为多发肿瘤。其他基线资料见表1。
2.2 术后早期复发的危险因素
单因素Logistic回归分析显示肿瘤最大径、肿瘤数目、γ-G T、NLR 与术后早期复发有关(均P<0.05)。多因素Logistic回归分析显示肿瘤数目(HR=2.737,9 5% C I=1.63 ~7.047,P=0.37)、γ-GT(HR=1.016,95% CI= 1.008~1.024,P<0.001)、NLR(HR=1.355,95% CI=1.013~1.812,P=0.041)为肝细胞癌术后早期复发的独立危险因素(表2)。
表1 225例肝细胞癌患者的基线资料
Table 1 Baseline characteristics of the 225 HCC patients
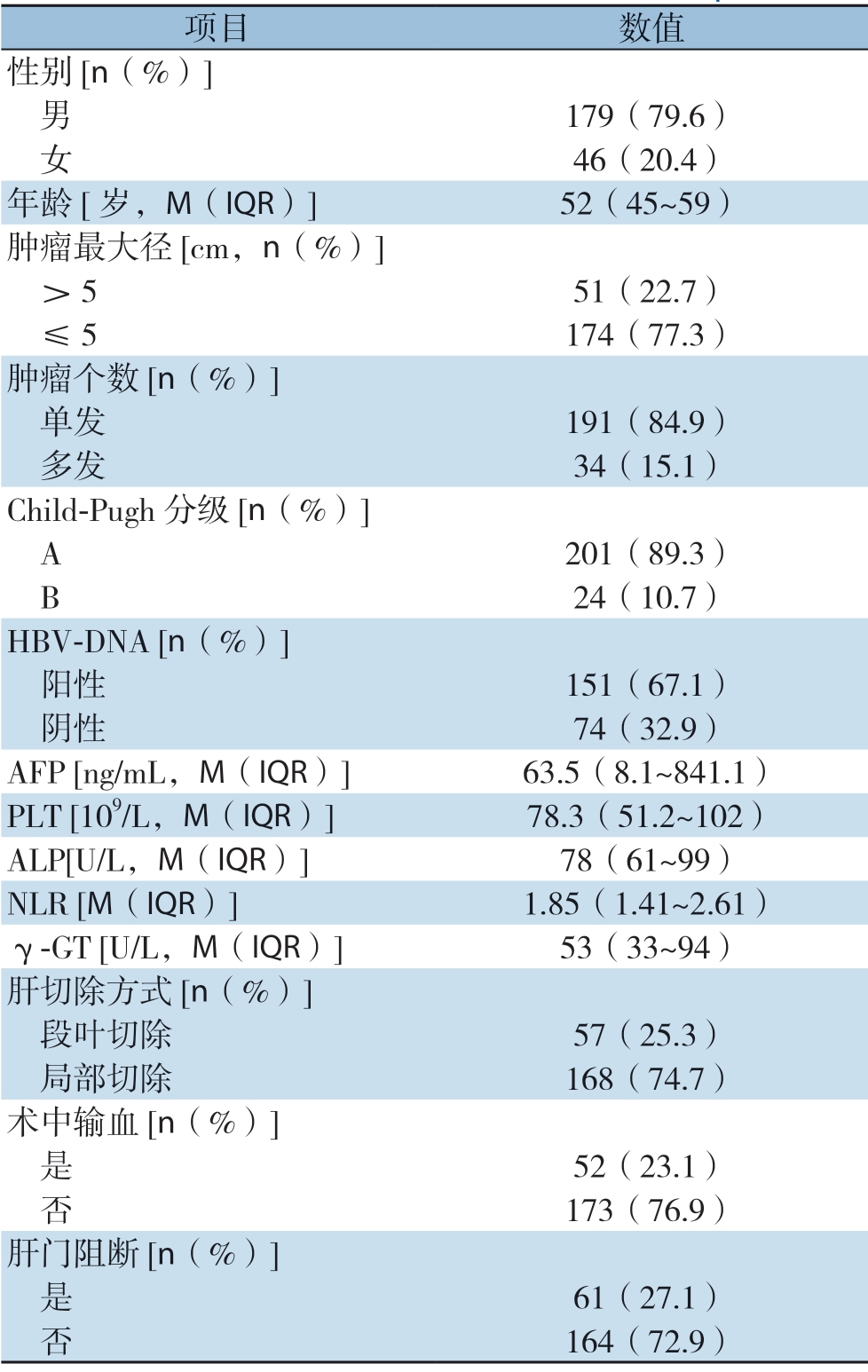
项目 数值性别[n(%)] 男 179(79.6) 女 46(20.4)年龄[岁,M(IQR)] 52(45~59)肿瘤最大径[cm,n(%)] >5 51(22.7) ≤5 174(77.3)肿瘤个数[n(%)] 单发 191(84.9) 多发 34(15.1)Child-Pugh 分级[n(%)] A 201(89.3) B 24(10.7)HBV-DNA [n(%)] 阳性 151(67.1) 阴性 74(32.9)AFP [ng/mL,M(IQR)] 63.5(8.1~841.1)PLT [109/L,M(IQR)] 78.3(51.2~102)ALP[U/L,M(IQR)] 78(61~99)NLR [M(IQR)] 1.85(1.41~2.61)γ-GT [U/L,M(IQR)] 53(33~94)肝切除方式[n(%)] 段叶切除 57(25.3) 局部切除 168(74.7)术中输血[n(%)] 是 52(23.1) 否 173(76.9)肝门阻断[n(%)] 是 61(27.1) 否 164(72.9)
表2 影响肝细胞癌患者早期复发的单因素和多因素分析
Table 2 Univariate and multivariate analysis of factors for early recurrence in HCC patients
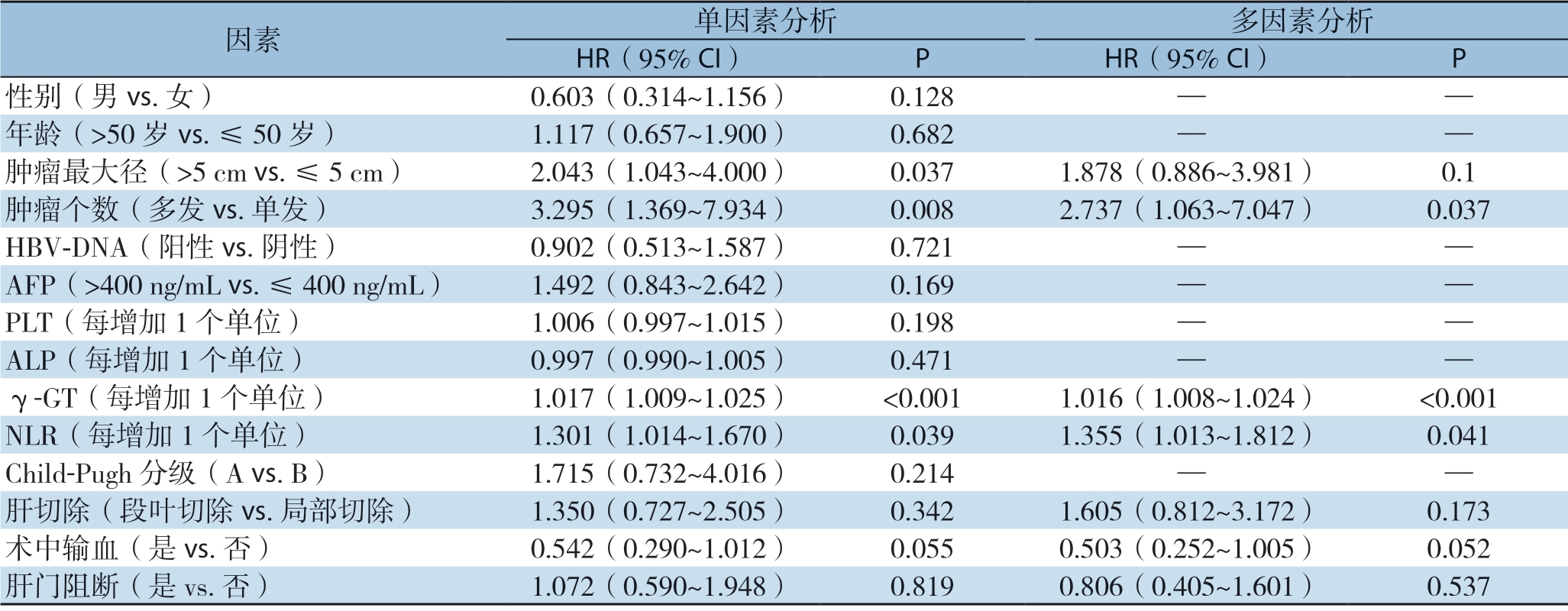
因素 单因素分析 多因素分析HR(95% CI) P HR(95% CI) P性别(男vs.女) 0.603(0.314~1.156) 0.128 — —年龄(>50 岁vs.≤50 岁) 1.117(0.657~1.900) 0.682 — —肿瘤最大径(>5 cm vs.≤5 cm) 2.043(1.043~4.000) 0.037 1.878(0.886~3.981) 0.1肿瘤个数(多发vs.单发) 3.295(1.369~7.934) 0.008 2.737(1.063~7.047) 0.037 HBV-DNA(阳性vs.阴性) 0.902(0.513~1.587) 0.721 — —AFP(>400 ng/mL vs.≤400 ng/mL) 1.492(0.843~2.642) 0.169 — —PLT(每增加1 个单位) 1.006(0.997~1.015) 0.198 — —ALP(每增加1 个单位) 0.997(0.990~1.005) 0.471 — —γ-GT(每增加1 个单位) 1.017(1.009~1.025) <0.001 1.016(1.008~1.024) <0.001 NLR(每增加1 个单位) 1.301(1.014~1.670) 0.039 1.355(1.013~1.812) 0.041 Child-Pugh 分级(A vs.B) 1.715(0.732~4.016) 0.214 — —肝切除(段叶切除vs.局部切除) 1.350(0.727~2.505) 0.342 1.605(0.812~3.172) 0.173术中输血(是vs.否) 0.542(0.290~1.012) 0.055 0.503(0.252~1.005) 0.052肝门阻断(是vs.否) 1.072(0.590~1.948) 0.819 0.806(0.405~1.601) 0.537
2.3 γ-GT、NLR 预测早期复发的ROC 曲线分析
γ-GT预测预后的AUC为67.6%(95% CI= 60.7%~74.5%),最佳截断值为108 U/L,其敏感度和特异度分别为34.6%和96.8%。NLR预测预后的AUC为59.1%(95% CI=51.6%~66.6%),最佳截断值为2.11,其敏感度和特异度分别为5 2.3%和6 5.3%。利用SPSS软件建立γ-GT和NLR联合指标,该指标AUC为70.4%(95% CI= 63.6%~77.1%),最佳截断值为0.57,其敏感度和特异度分别为52.3%和81.1%。γ-GT&NLR联合指标预测肿瘤复发优于单一指标(图1)。
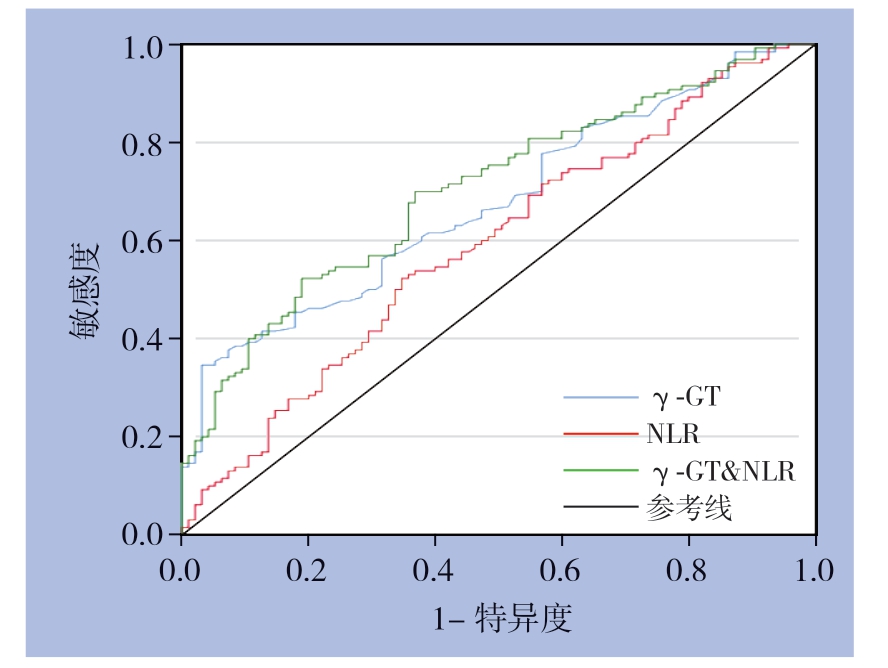
图1 γ-GT、NLR 以及γ-GT 与NLR 联合预测肝细胞癌患者术后早期复发的ROC 曲线
Figure 1 ROC curves of γ-GT, NLR and γ-GT plus NLR in predicting early postoperative recurrence in HCC patients
2.4 评分系统建立
分别将NLR 、γ-GT、肿瘤个数纳入回归方程, 得到复发风险函数:Y=0.254X1+0.016X2+0.973X3-1.390,X1、X2、X3分别代表NLR、γ-GT、肿瘤数目。根据HR值,将γ-GT>108 U/L赋值为1,NLR>2.11赋值为2,肿瘤个数>1赋值为3,否则赋值为0。可得危险评分界值为0 ~6 分。<3 分为低危组,≥3 分为高危组。Kaplan-Meier曲线分析低危组1、3、5年的无病生存率分别为76.9%、44.5%和32.8%,而高危组为33.4%、20.1%和6.69%,两组差异有统计学意义(P<0.001)(图2)。
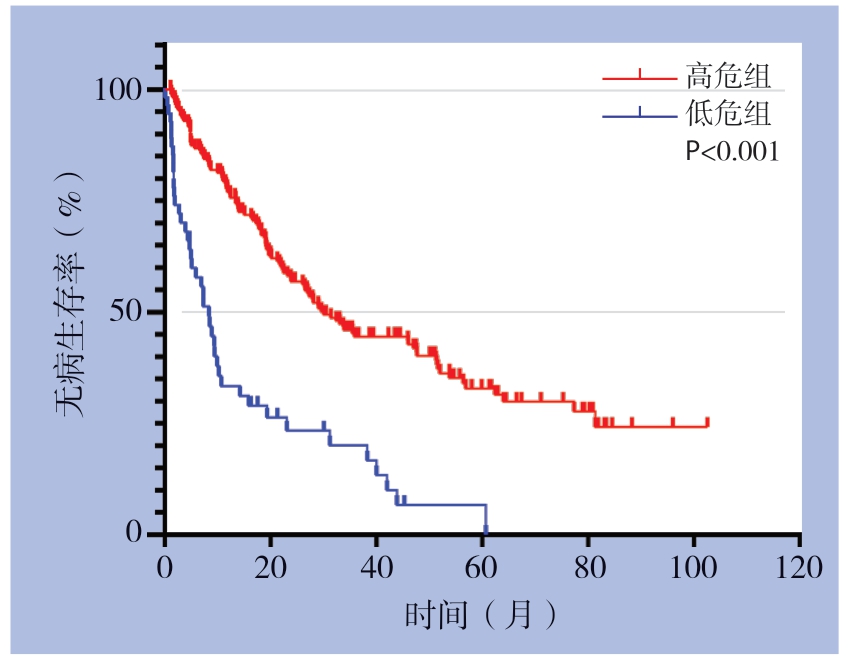
图2 高危组与低危组Kaplan-Meier 生存曲线
Figure 2 Kaplan-Meier survival curves for high-risk group and low-risk group
3 讨 论
肝细胞癌切除术后5年内复发率高达70%,并且大多数在术后2年内长出新的病灶[7]。早期复发(<2年)多表现为局部浸润、肝内播散和肿瘤自身生物学特征改变[8],肝癌术后早期复发是导致患者预后不良的重要因素。因此有效预测早期复发的指标将对患者的危险分层具有重要意义。
NLR 是癌症患者术后病死率的有效预测因子。术前NLR水平可以预测肝切除以及肝移植术后肝癌患者的无病生存率(disease-free survival,DFS)及总生存率(overall survival,OS)[9-10]。研究[11]表明中性粒细胞分泌大部分血管内皮生长因子,这是一种已知的参与肿瘤发展的促血管生成因子。此外,中性粒细胞通过产生精氨酸酶,一氧化氮和活性氧而抑制淋巴细胞、自然杀伤细胞和活化的T细胞的细胞毒活性,因此具有免疫抑制性[12]。由于肿瘤刺激,与肿瘤相关的巨噬细胞活性增加,导致中性粒细胞增多和/或淋巴细胞减少[13-15]。淋巴细胞介导抗肿瘤免疫,淋巴细胞减少提示肿瘤特异性T细胞数量减少[16]。NLR反映中性粒细胞与淋巴细胞的相对比例,高NLR水平意味着淋巴细胞少于中性粒细胞,淋巴细胞减少,可能导致淋巴细胞对恶性肿瘤的免疫反应较差,从而影响预后,增加肿瘤复发的风险[17-19]。另外,术前NLR值对微血管浸润(microvascular invasion,MVI)具有预测价值[20]。NLR截断值目前没有达成一致。Kim等[18]研究报道的NLR截断值为2.0,而Hung等[21]报道的NLR截断值为2.5。Gomez等[22]发现术前NLR≥5是无病生存期和总生存期的不良预测指标。本研究发现NLR是术后早期复发的独立危险因素,预测截断值为2.1。不同研究者确立的NLR截断值存在较大差异,这可能需要大样本、多中心、前瞻性研究来进一步探讨。
肝功能检查是广泛用于临床的血液测定,其中γ-GT的水平反映肝细胞的损伤程度,与细胞完整性或与胆道疾病有关[23]。一些研究[24-25]表明γ-GT升高可以造成DNA损伤、促进肿瘤进展、转移,提示恶性肿瘤患者的无病生存期较短[26-28]。此外,肝切除后血清γ-GT水平能预测肝癌患者的预后[29-30]。本研究发现术前血清γ-GT是术后早期复发的独立危险因素,其预测截断值为108 U/L。
联合NLR和γ-GT可用作肝癌的早期复发的预测因子,可提高单一指标的预测能力。为了减少混杂因素影响肝细胞癌患者的生存和复发,本研究将其他危险因素(如肿瘤大小,肿瘤数目等)纳入Logistic回归分析,发现肿瘤是否多发也是影响预后的重要因素。肿瘤数目越多,提示肿瘤呈多中心发展,因而这类患者更易复发和转移[31]。相较于影像学检查成本昂贵、具有辐射危害、肝硬化背景下微小结节性质难定,NLR、γ-GT是血常规和肝功能的常见指标,简单易得,再联合肿瘤数目,可对肝细胞癌患者早期复发进行危险分层。
本研究有几个局限性需要解决。一方面,由于NLR、γ-GT值是一个动态变化的过程,本研究仅使用基线值来预测结果,因此可能会遗漏很多信息。另外本文是一篇回顾性研究,且样本量较小,可能存在选择偏倚。本研究结果仍需要大规模、多中心前瞻性研究来验证。
[1] Torre LA, Bray F, Siegel RL, et al.Global cancer statistics, 2012[J].CA Cancer J Clin, 2015, 65(2):87-108.doi:10.3322/caac.21262.
[2] Chen W, Zheng R, Baade PD, et al.Cancer statistics in China, 2015[J].CA Cancer J Clin, 2016, 66(2):115-132.doi:10.3322/caac.21338.
[3] Shim JH, Jun MJ, Han S, et al.Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma[J].Ann Surg, 2015, 261(5):939-946.doi:10.1097/SLA.0000000000000747.
[4] Imamura H, Matsuyama Y, Tanaka E, et al.Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy[J].J Hepatol, 2003, 38(2):200-207.doi: 10.1016/s0168-8278(02)00360-4.
[5] Wu JC, Huang YH, Chau GY, et al.Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma[J].J Hepatol, 2009, 51(5):890-897.doi:10.1016/j.jhep.2009.07.009.
[6] European Association for the Study of the Liver.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J].J Hepatol, 2018, 69(1):182-236.doi:10.1016/j.jhep.2018.03.019.
[7] Dhir M, Melin AA, Douaiher J, et al.A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma[J].Ann Surg, 2016, 263(6):1112-1125.doi:10.1097/SLA.0000000000001556.
[8] Poon RT, Fan ST, Ng IO, et al.Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma[J].Cancer, 2000, 89(3):500-507.
[9] Okamura Y, Sugiura T, Ito T, et al.Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma[J].Br J Surg, 2016, 103(7):891-898.doi:10.1002/bjs.10123.
[10] Liao W, Zhang J, Zhu Q, et al.Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection[J].Transl Oncol, 2014, 7(2):248-255.doi:10.1016/j.tranon.2014.02.011.
[11] Kusumanto YH, Dam WA, Hospers GAP, et al.Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor[J].Angiogenesis, 2003, 6(4):283-287.doi: 10.1023/B:AGEN.0000029415.62384.ba.
[12] Müller I, Munder M, Kropf P, et al.Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms?[J].Trends Immunol, 2009, 30(11):522-530.doi:10.1016/j.it.2009.07.007.
[13] Mano Y, Shirabe K, Yamashita Y, et al.Preoperative neutrophil-tolymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis[J].Ann Surg, 2013, 258(2):301-305.doi:10.1097/SLA.0b013e318297ad6b.
[14] Pollard JW.Tumour-educated macrophages promote tumour progression and metastasis[J].Nat Rev Cancer, 2004, 4(1):71-78.doi:10.1038/nrc1256.
[15] Coussens LM, Werb Z.Inflammation and cancer[J].Nature, 2002, 420(6917):860-867.doi: 10.1038/nature01322.
[16] Schreiber RD, Old LJ, Smyth MJ.Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion[J].Science, 2011, 331(6024):1565-1570.doi: 10.1126/science.1203486.
[17] Motomura T, Shirabe K, Mano Y, et al.Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment[J].J Hepatol, 2013, 58(1):58-64.doi:10.1016/j.jhep.2012.08.017.
[18] Kim WJ, Lim TW, Park PJ, et al.Prognostic markers affecting the early recurrence of hepatocellular carcinoma with liver cirrhosis after curative resection[J].Int J Biol Markers, 2019, 34(2):123-131.doi:10.1177/1724600819834306.
[19] 邱康华.中性粒细胞/淋巴细胞比值与肝脏疾病关系的临床研究进展[J].赣南医学院学报, 2019, 39(1):101-104.doi:10.3969/j.issn.1001-5779.2019.01.027.
Qiu KH.Clinical research progress in the relationship between neutrophil-to-lymphocyte ratio and liver diseases[J] Journal of Gannan Medical University, 39(1):101-104.doi:10.3969/j.issn.1001-5779.2019.01.027.
[20] 任家书, 马秀现, 李健, 等.术前中性粒细胞/淋巴细胞比值对小肝癌患者并发微血管浸润的预测价值[J].中国普通外科杂志, 2018, 27(7):840-846.doi:10.3978/j.issn.1005-6947.2018.07.007.
Ren JS, Ma XX, Li J, et al.Value of preoperative neutrophil to lymphocyte ratio for prediction of microvascular invasion in patients with small hepatocellular carcinoma[J] Chinese Journal of General Surgery, 2018, 27(7):840-846.doi:10.3978/j.issn.1005-6947.2018.07.007.
[21] Hung HC, Lee JC, Cheng CH, et al.Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection[J].J Hepatobiliary Pancreat Sci, 2017, 24(10):559-569.doi:10.1002/jhbp.498.
[22] Gomez D, Farid S, Malik HZ, et al.Preoperative neutrophil-tolymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma[J].World J Surg, 2008, 32(8):1757-1762.doi:10.1007/s00268-008-9552-6.
[23] Whitfield JB.Gamma glutamyl transferase[J].Crit Rev Clin Lab Sci, 2001, 38(4):263-355.doi: 10.1080/20014091084227.
[24] Corti A, Duarte TL, Giommarelli C, et al.Membrane gammaglutamyl transferase activity promotes iron-dependent oxidative DNA damage in melanoma cells[J].Mutat Res, 2009, 669(1-2):112-121.doi:10.1016/j.mrfmmm.2009.05.010.
[25] Kunutsor SK.Gamma-glutamyltransferase-friend or foe within?[J].Liver Int, 2016, 36(12):1723-1734.doi:10.1111/liv.13221.
[26] Hanigan MH.Gamma-glutamyl transpeptidase: redox regulation and drug resistance[J].Adv Cancer Res, 2014, 122:103-141.doi:10.1016/B978-0-12-420117-0.00003-7.
[27] Luo C, Xu B, Fan Y, et al.Preoperative Gamma-Glutamyltransferase Is Associated with Cancer-Specific Survival and Recurrence-Free Survival of Nonmetastatic Renal Cell Carcinoma with Venous Tumor Thrombus[J].Biomed Res Int, 2017, 2017:3142926.doi:10.1155/2017/3142926.
[28] Grimm C, Hofstetter G, Aust S, et al.Association of gammaglutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer[J].Br J Cancer, 2013, 109(3):610-614.doi:10.1038/bjc.2013.323.
[29] Nagasue N, Ono T, Yamanoi A, et al.Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis[J].Br J Surg, 2001, 88(4):515-522.doi: 10.1046/j.1365-2168.2001.01732.x.
[30] 赵杰, 余海波, 朱运海, 等.肝癌根治术前GGT、ALT/AST和术后临床病理特征与预后的关系[J].中华普通外科杂志, 2019, 34(4):328-331.doi:10.3760/cma.j.issn.1007-631X.2019.04.011.
Zhao J, Yu HB, Zhu YH, et al.Preoperative GGT, ALT/AST relates to clinicopathological features and prognosis after radical surgery for hepatocellular carcinoma[J].Zhong Hua Pu Tong Wai Ke Za Zhi, 2019, 34(4):328-331.doi:10.3760/cma.j.issn.1007-631X.2019.04.011.
[31] 乐琪, 朱同恩, 莫志远, 等.原发性肝癌患者手术切除术后早期复发影响因素分析[J].中国普通外科杂志, 2019, 28(1):18-23.doi:10.7659/j.issn.1005-6947.2019.01.003.
Le Q, Zhu TE, Mo ZY, et al.Analysis of factors for early recurrence of patients with hepatocellular carcinoma after resection[J].Chinese Journal of General Surgery, 2019, 28(1):18-23.doi:10.7659/j.issn.1005-6947.2019.01.003.
