乳腺癌是全世界妇女最常见的恶性肿瘤之一[1]。三阴性乳腺癌(triple-negative breast cancer,TNBC)是乳腺癌的一种亚型,不表达HER-2 受体、雌激素受体(ER)及孕激素受体(PR)。临床上采取综合治疗方法,对早期TNBC的治疗可以起到一定的效果,而对于中期或晚期患者则以化学疗法为主[2]。但晚期患者化疗的反应期和总体生存期(OS)都较短,且易产生毒性和耐药性[3-6]。尽管TNBC患者仅占乳腺癌患者的12%~17%[7],但与其他类型的乳腺癌相比,TNBC是侵袭性和转移性最强的亚型,且由于缺乏有效的治疗靶点而被认为是预后最差的亚型[8-9]。毫无疑问,研发更为有效的分子靶标和新型生物标志物是完善TNBC治疗和监测的当务之急。新标靶的探索及相关机制对治疗TNBC具有重要意义。
最近,环状RNA(circular RNA,circRNA)对癌症进展和复发的复杂影响引起了医学界极大的研究兴趣[10]。circRNA是一类内源单链非编码RNA分子,在人类细胞中广泛分布且种类,可以调节真核生物中的基因表达[11]。circRNA作为一种调控性RNA,可以调控基因转录,调节选择性剪接,抑制miRNA的成熟,促进蛋白质与蛋白质的相互作用以及充当miRNA的海绵[12]。它是一种稳定表达的非编码RNA,circRNA的失调在人类癌症发病机理中也起着至关重要的作用[13]。越来越多的报道表明circRNA与多种人类肿瘤相关,例如乳腺癌[14],肺癌[15],肝细胞癌[16],膀胱癌[17],肾细胞癌[18]和乳腺癌[19]。有研究报道,来自F-box和WD重复结构域的circRNA(circFBXW7)通过编码新型21 kDa蛋白FBXW7-185aa在人脑中的发挥肿瘤抑制因子的作用。基因FBXW7部分外显子来源的circRNA编码的蛋白可与FBXW7 mRNA编码的蛋白协同作用,调控原癌基因Myc的稳定性,从而抑制胶质瘤的发生和进展[20]。最近,一项研究认为circFBXW7可通过竞争性吸附miR-197-3p并编码FBXW7-185aa蛋白上调FBXW7基因表达,从而抑制乳腺癌的进展[21]。然而,circFBXW7在TNBC的作用及机制仍不清楚。本研究旨在探讨circFBXW7对TNBC细胞的增殖、侵袭性的影响。
1 材料与方法
1.1 临床标本与资料
临床标本收集了从2005年3月3日—2011年9月26日于中山大学肿瘤防治中心被确认为TNBC患者的240例新鲜肿瘤样本,患者的随访时间5年以上或者截止到死亡结局发生时。所有切除的组织均立即浸入RNAlater(Ambion,TX)中;患者定期随访,并记录临床数据。这项研究经中山大学肿瘤防治中心伦理委员会批准,并根据赫尔辛基宣言进行。参与本研究前已获得所有患者书面知情同意。240例患者的基本临床资料见表1。
表1 240 例患者的基本临床资料[n(%)]
Table 1 The general data of the 240 patients[n(%)]
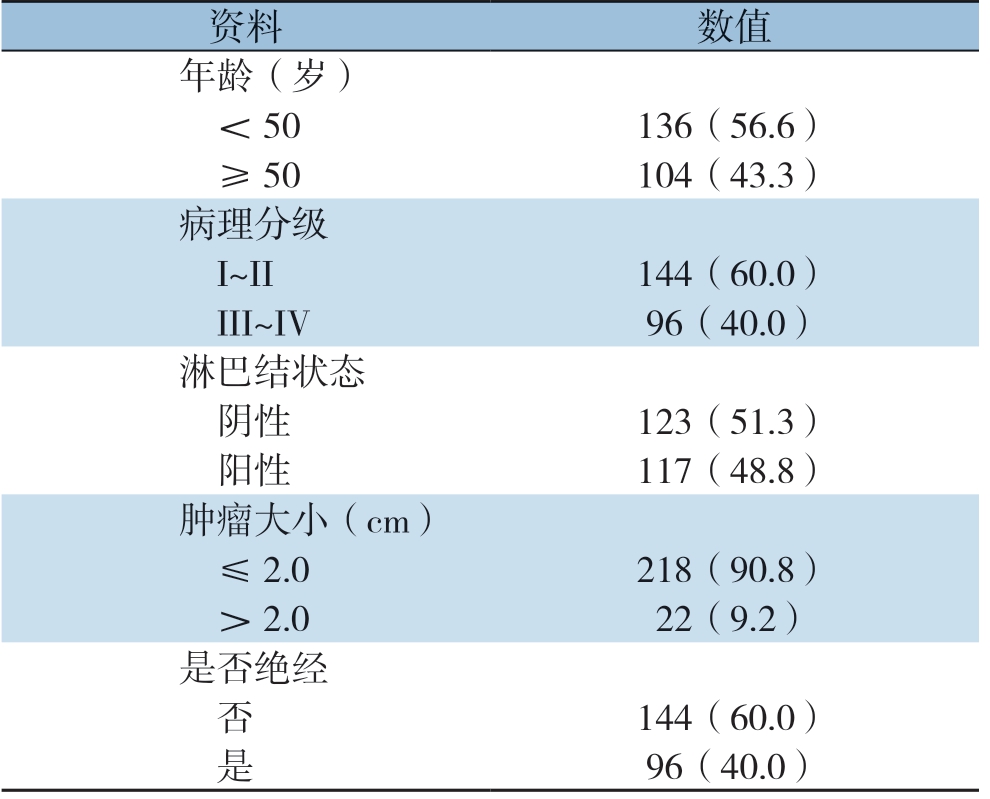
资料数值年龄(岁)<50136(56.6)≥50104(43.3)病理分级I~II144(60.0)III~IV96(40.0)淋巴结状态阴性123(51.3)阳性117(48.8)肿瘤大小(cm)≤2.0218(90.8)>2.022(9.2)是否绝经否144(60.0)是96(40.0)
1.2 细胞株及主要试剂
人类TNBC细胞株MDA-MB-231、HCC1806购自美国ATCC细胞库,经过适当培养并传代不超过10代。MDA-MB231和HCC1806细胞系均无支原体及其他污染物感染。RPMI1640和DMEM(4.5 g)培养基、胰蛋白酶、胎牛血清(FBS)购自美国Gibco公司,转染试剂Lipofectamine 2000,TRIzol购自美国Invitrogen公司,SYBRSuper Mix试剂购自日本Takara,PCR引物购自GeneCopoeia有限公司。CCK-8试剂盒购自日本Dojindo,Transwell小室(孔径3.0 μm)购自美国Corning公司,Matrigel基质胶购自美国BD公司。circFBXW7 shRNA购自GeneCopoeia有限公司。
1.3 qRT-PCR 法检测circFBXW7 在TNBC 组织中的表达
乳腺癌组织和细胞用按照试剂说明书用TRIzol裂解提取总RNA,按照GeneCopoeia反转录试剂盒说明书制备cDNA,用SYBRPremix Ex Taq进行qRT-PCR。以GAPDH为内参。利用各实验组目的基因及内参基因的Ct值,按照公式ΔCt=Ct目的基因-Ct内参基因,以2-ΔΔCt法计算目的基因的相对表达量,所有qRT-PCR分析均使用Bio-Rad IQTM5多色实时荧光定量PCR检测系统(美国)进行。反应体系为20 µL,每个样本重复3次。
1.4 载体构建和转染
载体的合成和转染人circ FBXW7 的全长cDNA,并将其克隆到pCDNA3.0 载体中以构建过表达质粒,通过q RT-PCR 评估效率,用Lipofectamine 2000进行转染,完全培养基中细胞生长至约50%密度;室温下孵育20 min,使形成脂质体复合体;往复合体中加入无血清的培养液,温和混匀,加入到待转染的培养瓶中;细胞培养箱孵育24~48 h后,收集细胞,抽提总RNA,蛋白。microRNA抑制剂和模拟物由GeneCopoeia公司合成。
1.5 CCK-8 检测细胞增殖
制备单细胞悬液:取对数增殖期的MDAMB-231和HCC1806以103细胞/孔的密度接种在96孔板中,每孔设置3个复孔,孵育48 h后。每个孔中加入10 µLCCK-8溶液添加至铺板的细胞中,将其在37 ℃,5%CO2的潮湿环境中培养,孵育2 h。使用微量滴定板读数器评估450 nm波长的吸光度。
1.6 Transwell 小室检测细胞迁移与侵袭能力
通常,使用迁移室进行Transwell分析,收集稳定转染的细胞,以2×104个悬浮细胞重悬于无血清的培养基中,每孔设置3个复孔,然后转移到水合基质胶室中。并将20%胎牛血清作为趋化剂添加到下室中在37 ℃和5%CO2孵育48 h后,用棉签刮擦上表面的细胞,将膜底部的浸润细胞在室温下用甲醇固定30 min,然后在室温下用结晶紫染色,计数并成像。
1.7 克隆形成实验
消化重悬细胞至适宜密度,以1×103个细胞每孔的数量接种于六孔板中并在温箱中孵育7~14 d,待肉眼可观察到细胞集落形成终止培养。用PBS洗去残余的培养基后,甲醇固定细胞集落10 min,倒去甲醇开盖晾干剩余的甲醇,加入0.1%结晶紫染色15 min。之后,对集落进行拍照和计数。
1.8 统计学处理
本实验数据应用SPSS16.0软件进行统计分析,各组之间的比较采用t检验和χ2检验进行,根据Kaplan-Meier方法绘制OS和无病生存(DFS)曲线,并使用Log-rank检验进行比较,从手术当天起开始计算存活时间。使用Cox回归分析circFBXW7单因素和多因素与生存的关系,以及风险值(HR)。P<0.05为差异有统计学意义。
2 结果
2.1 circFBXW7 表达与TNBC 患者预后的关系
240 例TNBC 患者新鲜癌组织标本中circ FBXW7 的平均表达水平为1.043±0.268。以平均表达水平定义为临界值,将患者分为高表达组(表达量>1.043)和低表达组(表达量≤1.043),其中高表达组100例,低表达组140例,低表达组死亡为28 例,复发为30 例;高表达组死亡10例,复发12例。Kaplan-Meier生存分析显示,低circFBXW7组患者的OS和DFS均明显短于高circFBXW7组患者(均P<0.05)(图1)。
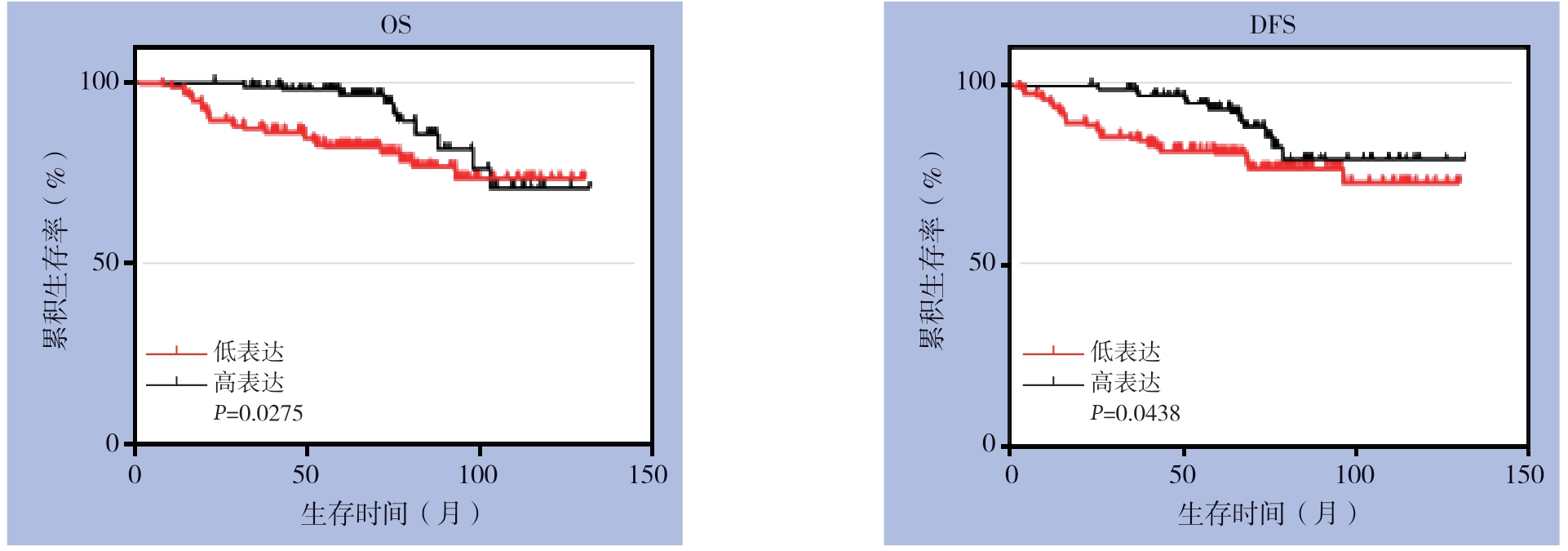
图1 不同circFBXW7 表达水平TNBC 患者的OS 和DFS 曲线
Figure 1 The OS and DFS curves of TNBC patients with different circFBXW7 expression levels
2.2 TNBC 患者预后因素分析
单因素Cox回归分析显示,组织学分级、肿瘤大小、淋巴结转移、TNM分期、circFBXW7表达是TNBC患者预后的影响因素(均P<0.05);多因素Cox回归分析显示,组织学分级、TNM分期、circFBXW7表达是TNBC患者预后的独立影响因素(均P<0.05)(表2)。
表2 单因素和多因素Cox 回归分析
Table 2 Univariate and multivariate Cox regression analysis
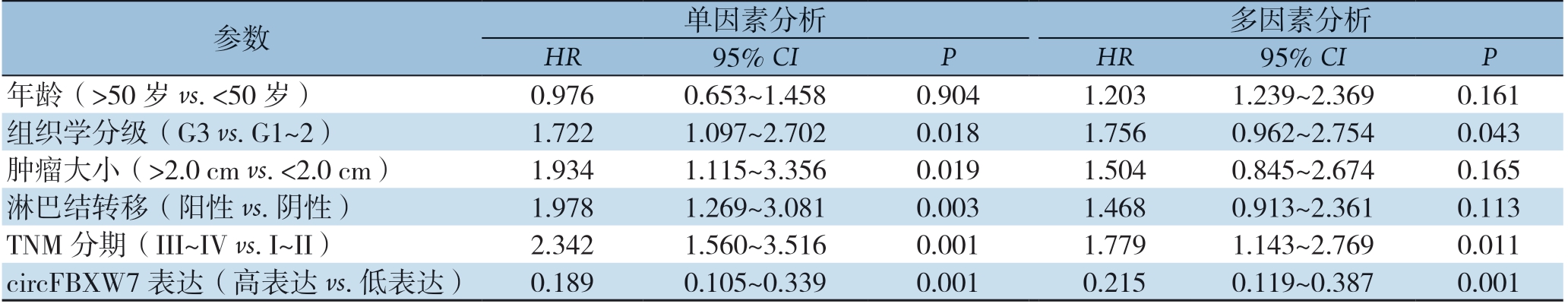
参数50 岁)单因素分析多因素分析HR 95% CIP HR 95% CIP年龄(>50 岁vs.<0.9760.653~1.4580.9041.2031.239~2.3690.161组织学分级(G3 vs.G1~2) 1.7221.097~2.7020.0181.7560.962~2.7540.043肿瘤大小(>2.0 cm vs.<2.0 cm) 1.9341.115~3.3560.0191.5040.845~2.6740.165淋巴结转移(阳性vs.阴性) 1.9781.269~3.0810.0031.4680.913~2.3610.113TNM 分期(III~IV vs.I~II) 2.3421.560~3.5160.0011.7791.143~2.7690.011 circFBXW7 表达(高表达vs.低表达) 0.1890.105~0.3390.0010.2150.119~0.3870.001
2.3 circFBXW7 对TNBC 细胞增殖的影响
为了研究circFBXW7在TNBC细胞中的生物学功能和作用,构建了circFBXW7过表达载体,并验证了其在MDA-MB-231和HCC1806细胞系中的作用,结果显示,过表达组TNBC细胞株(MDAMB-231、HCC1806)的circFBXW7表达量明显高于对照组(均P<0.05)(图2)。细胞增殖测定实验结果表明,circFBXW7的过表达的TNBC细胞系(MDA-MB-231和HCC1806)的增殖能力明显降低(均P<0.05)(图3)。
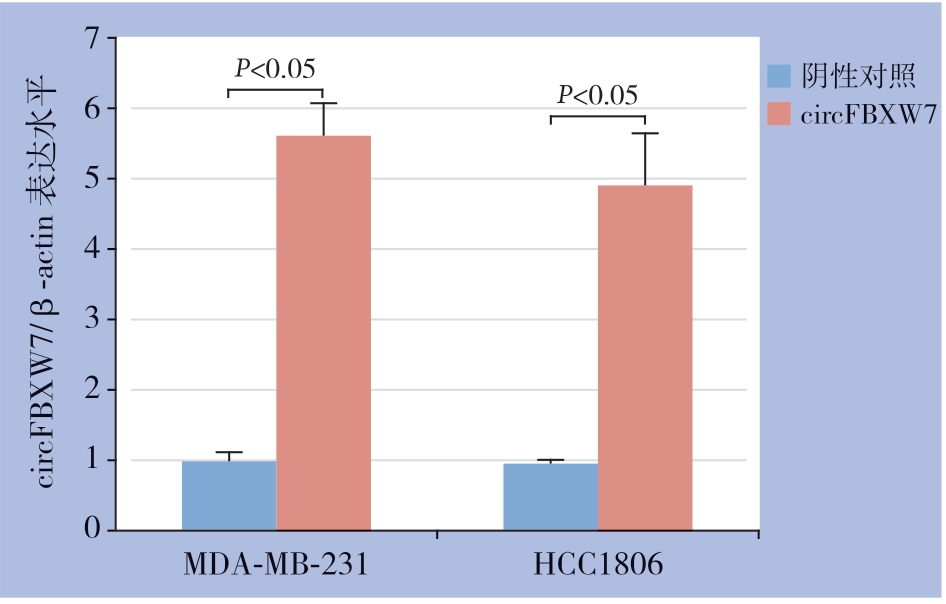
图2 circFBXW7 在TNBC 细胞株中过表达验证
Figure 2 Verification of circFBXW7 over-expression of in TNBC cell lines
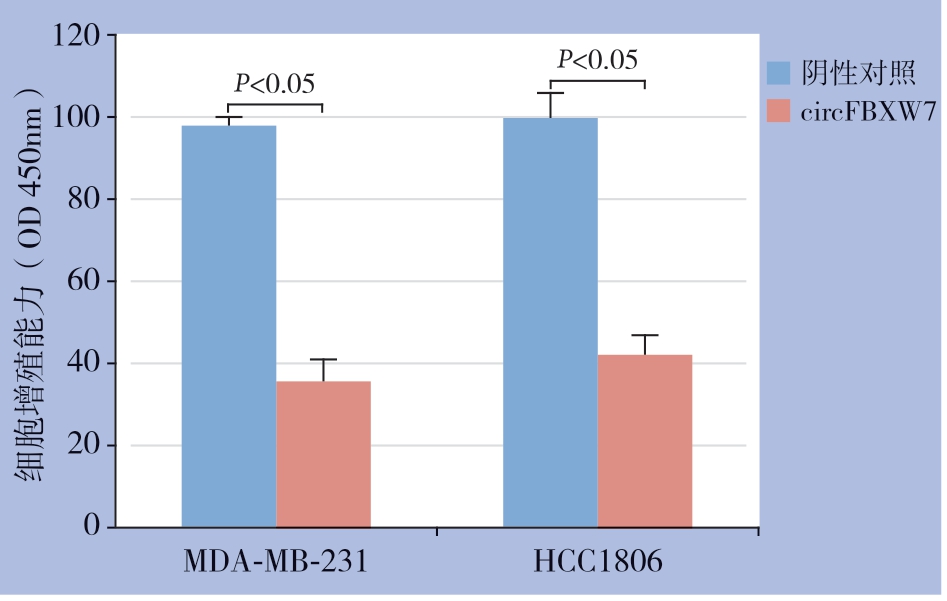
图3 过表达circFBXW7 对TNBC 细胞增殖能力的影响
Figure 3 Effect of circFBXW7 over-expression on proliferation of TNBC cells
2.4 circFBXW7 对TNBC 细胞的迁移、侵袭的影响
Transwell实验结果显示,circFBXW7的过表达明显抑制了这两种TNBC细胞系的迁移能力(均P<0.05)(图4),加入基质胶后检测过表达circFBXW7后两种TNBC细胞系的侵袭能力(均P<0.05)(图5)。
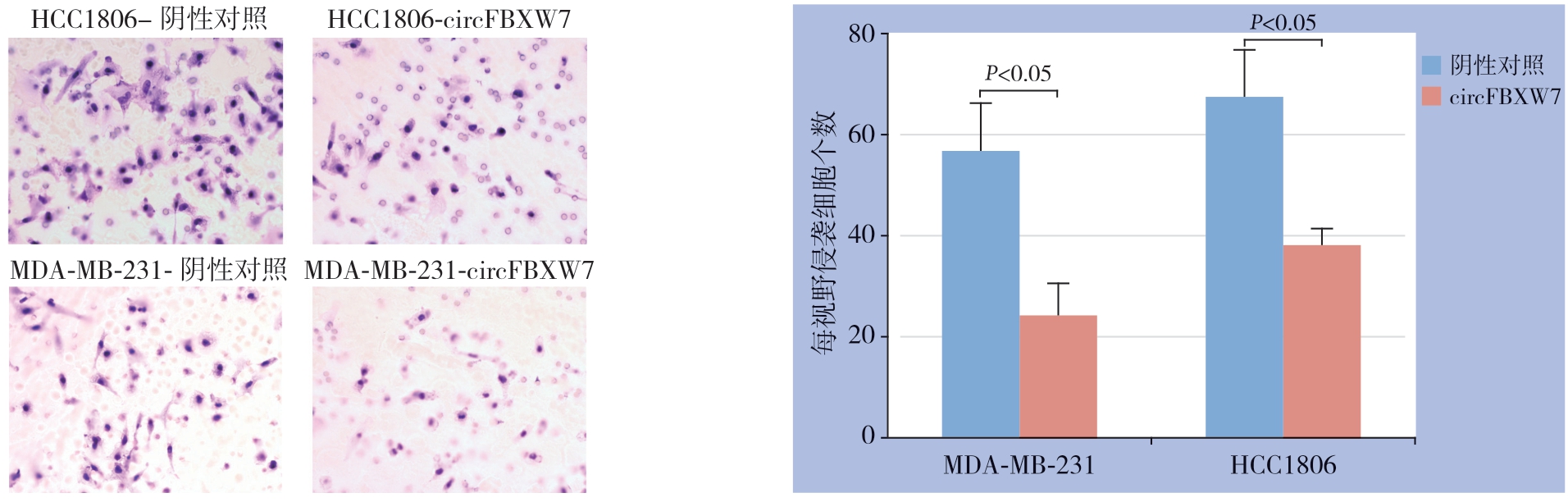
图4 过表达circFBXW7 对TNBC 细胞迁移能力的影响
Figure 4 Effect of circFBXW7 over-expression on migration ability of TNBC cells
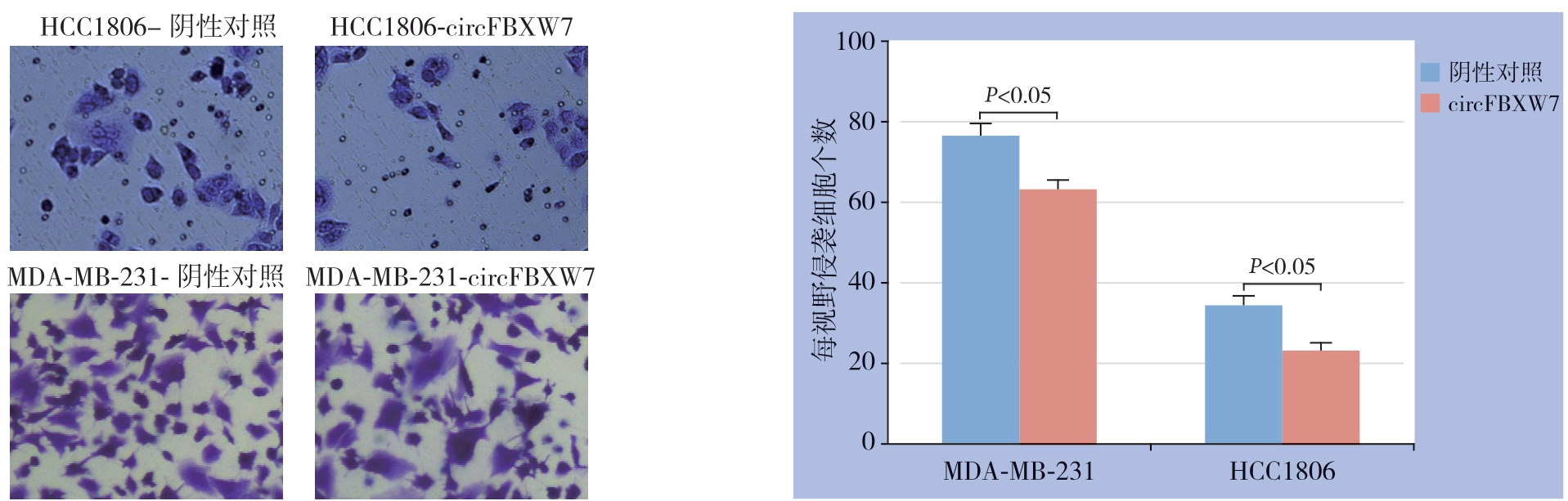
图5 过表达circFBXW7 对TNBC 细胞侵袭能力的影响
Figure 5 Effect of circFBXW7 over-expression on invasion ability of TNBC cells
2.5 过表达circFBXW7 对miR-197-3p 表达的影响
q RT-PCR 结果显示,过表达组TNBC 细胞(MDA-MB-231、HCC1806)中的miR-197-3p表达量明显低于对照组(均P<0.05)(图6)。
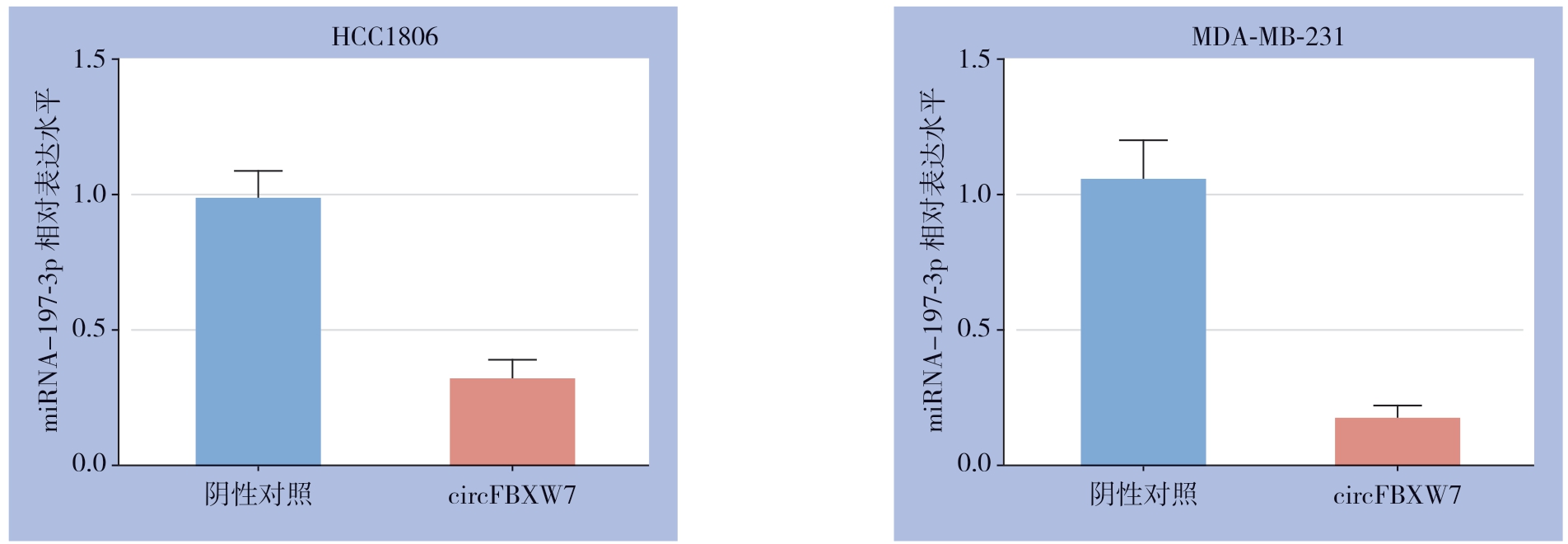
图6 过表达circFBXW7 对miR-197-3p 表达的影响
Figure 6 Influence of circFBXW7 overexpression on miRNA-197-3p expression
2.6 抑制miR-197-3p 对circFBXW7 敲除所致的细胞增殖和侵袭能力增强的影响
在敲除circ FBXW7 的细胞中转染miR-197-3p抑制剂,再进行Transwell实验和克隆形成实验,结果显示,抑制miR-197-3 p 后可减少由于circ FBXW7 敲低所致的细胞增殖和侵袭能力的增强(均P<0.05)(图7)。该结果提示,circFBXW7可能是通过miR-197-3p发挥作用,通过吸附miR-197-3 p,减少其表达,抑制肿瘤的发展。
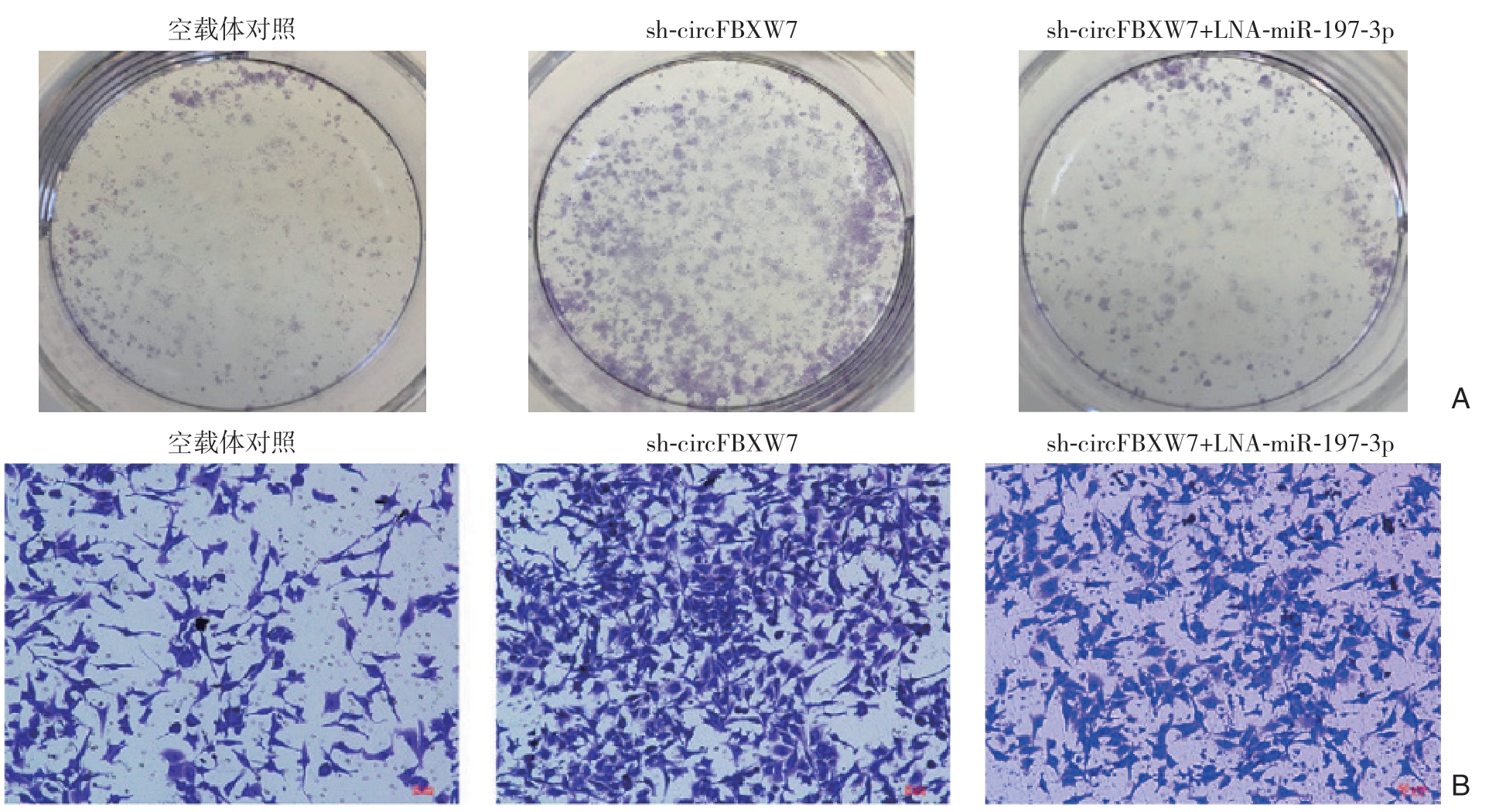
图7 抑制miR-197-3p 对circFBXW7 敲除所致的细胞增殖和侵袭能力增强的影响 A:克隆形成实验检测增殖能力;B:Transwell 实验检测侵袭能力
Figure 7 Influences of miR-197-3p inhibition on the increased proliferation and invasion abilities induced by circFBXW7 knockdown A:Colony formation assay for determining proliferation ability;B:Transwell assay for determining invasion ability
3 讨论
尽管诊断和治疗手段得到了一定的发展,但是乳腺癌仍然是女性人群癌症中死亡的主要原因[22]。circRNA是人类癌症中重要的调节因子[23]。随着下一代测序和生物信息技术的迅速发展,人们已经阐明了circRNA在人类癌症中起着至关重要的作用,并表现出作为生物标志物和治疗靶标的巨大潜力[24-26]。circRNA与传统的线性RNA相比,通过反向剪接产生的共价闭合环没有5'-cap或3'-poly(A)尾巴,属于内源性非编码RNA的新兴亚组。其特征是组织特异性和结构稳定性[27-30]。迄今为止,已有不同研究小组筛选和鉴定了多种与癌症(包括乳腺癌在内)相关的功能性circRNA[31]。这些circRNA在多种癌症中起癌基因或抑癌作用[32]。据报道[21,33]circFBXW7具有抑癌作用,在正常脑组织中大量表达,在神经胶质瘤中下调[20]。但是,TNBC中circFBXW7的生物学功能和作用仍然不清楚。本研究探讨了circFBXW7在TNBC中的作用机制及临床价值,使用Kaplan-Meier方法分析了240例TNBC患者的circFBXW7表达水平来评判OS和DFS。结果发现低circFBXW7水平的乳腺癌患者的OS和DFS较差。且Cox分析结果显示,高表达circFBXW7患者死亡的风险明显升高。这些结果表明circFBXW7的低表达与TNBC患者较差的临床预后密切相关。由此可以推断circFBXW7可能是TNBC患者的独立预后因素。
笔者在之前的研究中检测了TNBC 细胞系(MDA-MB-231 和HCC1806)中circ FBXW7的表达水平,其表达水平较低,通过过表达circFBXW7后,用CCK8法检测MDA-MB-231和HCC1806细胞增殖情况并使用Transwell小室法实验检测细胞的迁移能力。结果发现在体外试验中,过表达MDA-MB-231 和HCC1806 细胞中circFBXW7水平后,显著抑制了MDA-MB-231和HCC1806细胞的增殖、侵袭能力,同时,两种细胞的miR-197-3p表达水平也明显降低。然而,使用miR-197-3p抑制剂后,circFBXW7表达降低所致的细胞增殖和侵袭能力增强被明显抑制。以上结果提示,circFBXW7可能通过吸附miR-197-3p,减少其表达,抑制肿瘤的发展。
总之。本研究表明,circ FBXW7 可以抑制TNBC 的发生发展,其作用机制可能与通过miRNA海绵作用吸附miR-197-3p的作用有关,circFBXW7有望成为TNBC预后生物标志物及潜在治疗靶标。
[1]Cai Y,Zheng Y,Gu J,et al.Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78[J].Cell Death Dis,2018.9(6):636.doi:10.1038/s41419–018–0669–8.
[2]夏林玉,徐卫云.三阴性乳腺癌治疗的新进展[J].中国普通外科杂志,2016,25(5):741–746.doi:10.3978/j.issn.1005–6947.2016.05.020.
Xia LY,Xu WY.Treatment of triple-negative breast cancer:resent progress[J].Chinese Journal of General Surgery,2016,25(5):741–746.doi:10.3978/j.issn.1005–6947.2016.05.020.
[3]Tan AR,Wright GS,Thummala AR,et al.Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer:a multicentre,randomised,open-label,phase 2 trial[J].Lancet Oncol,2019,20(11):1587–1601.doi:10.1016/S1470–2045(19)30616–3.
[4]Pascual J,Turner NC.Targeting the PI3-kinase pathway in triplenegative breast cancer[J].Ann Oncol,2019,30(7):1051–1060.doi:10.1093/annonc/mdz133.
[5]Yang Fu N,Visvader JE.Halting triple negative breast cancer by targeting PROCR[J].Cell Res,2019,29(11):875–876.doi:10.1038/s41422–019–0245–5.
[6]Loh HY,Norman BP,Lai KS,et al.The Regulatory Role of MicroRNAs in Breast Cancer[J].Int JMol Sci,2019,20(19):4940.doi:10.3390/ijms20194940.
[7]曹希,徐雅莉,孙强.年龄与三阴性乳腺癌患者预后的关系[J].中国普通外科杂志,2020,29(5):515–524.doi:10.7659/ j.issn.1005–6947.2020.05.001.
Cao X,Xu YL,Sun Q.Relationship between age and prognosis in patients with triple-negative breast cancer[J].Chinese Journal of General Surgery,2020,29(5):515–524.doi:10.7659/j.issn.1005–6947.2020.05.001.
[8]杨柳,王殊.晚期三阴性乳腺癌的治疗进 展[J].中国普通外科杂志,2019,28(11):1342–1346.doi:10.7659/j.issn.1005–6947.2019.11.004.
Yang L,Wang S.Progress in treatment for advanced triple-negative breast cancer[J].Chinese Journal of General Surgery,2019,28(11):1342–1346.doi:10.7659/j.issn.1005–6947.2019.11.004.
[9]吴至佛,汪灵,黄俊辉.三阴性乳腺癌的生物标记物研究进展[J].中国普通外科杂志,2017,26(11):1472–1477.doi:10.3978/ j.issn.1005–6947.2017.11.016.
Wu ZF,Wang L,Huang JH.Research progress on biomarkers of triple-negative breast cancer[J].Chinese Journal of General Surgery,2017,26(11):1472–1477.doi:10.3978/j.issn.1005–6947.2017.11.016.
[10]Yin Y,Long J,He Q,et al.Emerging roles of circRNA in formation and progression of cancer[J].JCancer,2019,10(21):5015–5021.doi:10.7150/jca.30828.eCollection 2019.
[11]Zheng Q,Bao C,Guo W,et al.Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs[J].Nat Commun,2016,7:11215.doi:10.1038/ncomms11215.
[12]刘彦,周超,杨永学.ciRS-7/miR-7轴调控肿瘤 生长转移的研究进展[J].中国普通外科杂志,2015,24(7):1027–1031.doi:10.3978/j.issn.1005–6947.2015.07.020.
Liu Y,Zhou C,Yang YX,et al.Regulative role of ciRS-7/miR-7 axis in tumor growth and metastasis:recent advances[J].Chinese Journal of General Surgery,2015,24(7):1027–1031.doi:10.3978/j.issn.1005–6947.2015.07.020.
[13]Li RC,Ke S,Meng FK,et al.CiRS-7 promotes growth and metastasis of esophageal squamous cell carcinoma via regulation of miR-7/HOXB1[J].Cell Death Dis,2018,9(8):838.doi:10.1038/s41419–018–0852-y.
[14]Song H,Sun J,Kong W,et al.Construction of a circRNA-Related ceRNAPrognostic Regulatory Network in Breast Cancer[J].Onco Targets Ther,2020,13:8347–8358.doi:10.2147/OTT.S266507.
[15]Song J,Shi W,Gao,Z,et al.Downregulation of circRNA_100876Inhibited Progression of NSCLCIn Vitro via Targeting miR-636[J].Technol Cancer Res Treat,2020,19:1533033820951817.doi:10.1177/1533033820951817.
[16]彭婀敏,夏发达,王文龙,等.环状RNAFBLIM1在 肝细胞癌中生物学功能的初步研究[J].中国普通外科杂志,2019,28(9):1109–1114.doi:10.7659/j.issn.1005–6947.2019.09.012.
Peng EM,Xia FD,Wang WL,et al.Preliminary study of the biological function of circular RNAFBLIM1 in hepatocellular carcinoma[J].Chinese Journal of General Surgery,2019,28(9):1109–1114.doi:10.7659/j.issn.1005–6947.2019.09.012.
[17]Yu Q,Liu P,Han G,et al.CircRNA circPDSS1 promotes bladder cancer by down-regulating miR-16[J].Biosci Rep,2020,40(1):BSR20191961.doi:10.1042/BSR20191961.
[18]Chen Q,Liu T,Bao Y,CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway[J].Cancer Lett,2020,469:68–77.doi:10.1016/j.canlet.2019.10.017.
[19]Xue D,Wang H,Chen Y,et al.Circ-AKT3 inhibits clear cell renal cell carcinoma metastasis via altering miR-296–3p/E-cadherin signals[J].Mol Cancer,2019,18(1):151.doi:10.1186/s12943–019–1072–5.
[20]Yang Y,Gao X,Zhang M,et al.Novel Role of FBXW7Circular RNA in Repressing Glioma Tumorigenesis[J].JNatl Cancer Inst,2018,110(3):304–315.doi:10.1093/jnci/djx166.
[21]Ye F,Gao G,Zou Y,et al.circFBXW7Inhibits Malignant Progression by Sponging miR-197–3p and Encoding a 185-aa Protein in Triple-Negative Breast Cancer[J].Mol Ther Nucleic Acids,2019,18:88–98.doi:10.1016/j.omtn.2019.07.023.
[22]Verigos J,Karakaidos P,Kordias D,et al.The Histone Demethylase LSD1/KDM1AMediates Chemoresistance in Breast Cancer via Regulation of a Stem Cell Program[J].Cancers(Basel),2019,11(10):1585.doi:10.3390/cancers11101585.
[23]Feng Y,Yang Y,Zhao X,et al.Circular RNA circ0005276 promotes the proliferation and migration of prostate cancer cells by interacting with FUS to transcriptionally activate XIAP[J].Cell Death Dis,2019,10(11):792.doi:10.1038/s41419–019–2028–9.
[24]Cui Y,Li C,Zeng C,et al.Tongmai Yangxin pills antioxidative stress alleviates cisplatin-induced cardiotoxicity:Network pharmacology analysis and experimental evidence[J].Biomed Pharmacother,2018,108:1081–1089.doi:10.1016/j.biopha.2018.09.095.
[25]Su C,Gao X,Yang W,et al.Phosphorylation of Tudor-SN,a novel substrate of JNK,is involved in the efficient recruitment of Tudor-SN into stress granules[J].Biochim Biophys Acta Mol Cell Res,2017,1864(3):562–571.doi:10.1016/j.bbamcr.2016.12.018.
[26]Zou Y,Zheng S,Xiao W,et al.circRAD18 sponges miR-208a/3164 to promote triple-negative breast cancer progression through regulating IGF1 and FGF2 expression[J].Carcinogenesis,2019,40(12):1469–1479.doi:10.1093/carcin/bgz071.
[27]Zhao W,Cui Y,Liu L,et al.Splicing factor derived circular RNA circUHRF1 accelerates oral squamous cell carcinoma tumorigenesis via feedback loop[J].Cell Death Differ,2020,27(3):919–933.doi:10.1038/s41418–019–0423–5.
[28]Zhang L,Zhou Q,Qiu Q,et al.CircPLEKHM3 acts as a tumor suppressor through regulation of the miR-9/BRCA1/DNAJB6/KLF4/AKT1 axis in ovarian cancer[J].Mol Cancer,2019,18(1):144.doi:10.1186/s12943–019–1080–5.
[29]Zhao W,Ma X,Liu L,et al.SNHG20:A vital lncRNA in multiple human cancers[J].JCell Physiol,2019,doi:10.1002/jcp.28143.[Online ahead of print]
[30]Wu J,Qi X,Liu L,et al.Emerging Epigenetic Regulation of Circular RNAs in Human Cancer[J].Mol Ther Nucleic Acids,2019,16:589–596.doi:10.1016/j.omtn.2019.04.011.
[31]Han D,Li J,Wang H,et al.Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression[J].Hepatology,2017,66(4):1151–1164.doi:10.1002/hep.29270.
[32]Shang Q,Yang Z,Jia R,et al.The novel roles of circRNAs in human cancer[J].Mol Cancer,2019,18(1):6.doi:10.1186/s12943–018–0934–6.
[33]Liu J,Zhao K,Huang N,et al.Circular RNAs and human glioma[J].Cancer Biol Med,2019,16(1):11–23.doi:10.20892/j.issn.2095–3941.2018.0425.
